Easy and Efficient Recovery of EMIMCl from Cellulose Solutions by Addition of Acetic Acid and the Transition from the Original Ionic Liquid to an Eutectic Mixture
- PMID: 35164255
- PMCID: PMC8840234
- DOI: 10.3390/molecules27030987
Easy and Efficient Recovery of EMIMCl from Cellulose Solutions by Addition of Acetic Acid and the Transition from the Original Ionic Liquid to an Eutectic Mixture
Abstract
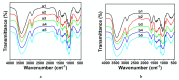
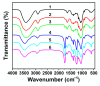
Ionic liquids (ILs) and deep eutectic solvents (DESs) are the two most widely used neoteric solvents. Recently, our group described how the simple addition of acetic acid (AcOH) to 1-Ethyl-3-methylimidazolium chloride (EMIMCl) could promote the transition from the original IL to an eutectic mixture of EMIMCl and AcOH. Herein, we studied how cellulose regeneration and EMIMCl recovery from EMIMCl solutions of cellulose could be benefited by the significant differences existing between EMIMCl- and EMIMCl·AcOH-based mixtures and the easy switching from one to the other. Finally, we also demonstrated that the transition could also be accomplished by addition of acetic anhydride and water so that the process could be eventually useful for the achievement of highly acetylated cellulose.
Keywords: cellulose acetylation; cellulose dissolution; cellulose regeneration; deep eutectic solvents; ionic liquid recovery; ionic liquids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wilkes J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002;4:73–80. doi: 10.1039/b110838g. - DOI
-
- Chatel G., Pereira J.F.B., Debbeti V., Wang H., Rogers R.D. Mixing ionic liquids—“simple mixtures” or “double salts”? Green Chem. 2014;16:2051–2083. doi: 10.1039/c3gc41389f. - DOI
-
- Kelley S.P., Narita A., Holbrey J.D., Green K.D., Reichert W.M., Rogers R.D. Understanding the Effects of Ionicity in Salts, Solvates, Co-Crystals, Ionic Co-Crystals, and Ionic Liquids, Rather than Nomenclature, Is Critical to Understanding Their Behavior. Cryst. Growth Des. 2013;13:965–975. doi: 10.1021/cg4000439. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

