Halogen-Bond Mediated [2+2] Photodimerizations: À la Carte Access to Unsymmetrical Cyclobutanes in the Solid State
- PMID: 35164313
- PMCID: PMC8839528
- DOI: 10.3390/molecules27031048
Halogen-Bond Mediated [2+2] Photodimerizations: À la Carte Access to Unsymmetrical Cyclobutanes in the Solid State
Abstract
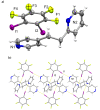
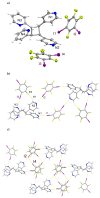
The ditopic halogen-bond (X-bond) donors 1,2-, 1,3-, and 1,4-diiodotetrafluorobenzene (1,2-, 1,3-, and 1,4-di-I-tFb, respectively) form binary cocrystals with the unsymmetrical ditopic X-bond acceptor trans-1-(2-pyridyl)-2-(4-pyridyl)ethylene (2,4-bpe). The components of each cocrystal (1,2-di-I-tFb)·(2,4-bpe), (1,3-di-I-tFb)·(2,4-bpe), and (1,4-di-I-tFb)·(2,4-bpe) assemble via N···I X-bonds. For (1,2-di-I-tFb)·(2,4-bpe) and (1,3-di-I-tFb)·(2,4-bpe), the X-bond donor supports the C=C bonds of 2,4-bpe to undergo a topochemical [2+2] photodimerization in the solid state: UV-irradiation of each solid resulted in stereospecific, regiospecific, and quantitative photodimerization of 2,4-bpe to the corresponding head-to-tail (ht) or head-to-head (hh) cyclobutane photoproduct, respectively.
Keywords: cocrystal; crystal engineering; cyclobutane; halogen bonding; photodimerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









Similar articles
-
Supramolecular Sandwiches: Halogen-Bonded Coformers Direct [2+2] Photoreactivity in Two-Component Cocrystals.Molecules. 2020 Feb 18;25(4):907. doi: 10.3390/molecules25040907. Molecules. 2020. PMID: 32085511 Free PMC article.
-
Halogen versus Hydrogen Bonding in Binary Cocrystals: Novel Conformation a Coformer with [2+2] Photoreactivity of Criss-Crossed C=C Bonds.Chemphyschem. 2020 Jan 16;21(2):154-163. doi: 10.1002/cphc.201900961. Epub 2019 Dec 9. Chemphyschem. 2020. PMID: 31600417
-
Crystal structure and photoreactivity of a halogen-bonded cocrystal based upon 1,2-diiodoperchlorobenzene and 1,2-bis(pyridin-4-yl)ethylene.Acta Crystallogr C Struct Chem. 2020 Jun 1;76(Pt 6):557-561. doi: 10.1107/S2053229620006233. Epub 2020 May 13. Acta Crystallogr C Struct Chem. 2020. PMID: 32499452
-
Structures and Reactivities of Cocrystals Involving Diboronic Acids and Bipyridines: In Situ Linker Reaction and 1D-to-2D Dimensionality Change via Crystal-to-Crystal Photodimerization.Chemistry. 2022 May 2;28(25):e202104604. doi: 10.1002/chem.202104604. Epub 2022 Mar 25. Chemistry. 2022. PMID: 35274391 Free PMC article.
-
A Divergent Alkyne Diol Directs [2 + 2] Photoreactivity in the Solid State: Cocrystal, Supramolecular Catalysis, and Sublimation Effects.Molecules. 2019 Aug 22;24(17):3059. doi: 10.3390/molecules24173059. Molecules. 2019. PMID: 31443541 Free PMC article.
Cited by
-
Chlorinated Anilines as Molecular Templates to Achieve [2 + 2] Cycloaddition Reactions within Organic Cocrystals.ACS Omega. 2025 May 21;10(21):21922-21928. doi: 10.1021/acsomega.5c01991. eCollection 2025 Jun 3. ACS Omega. 2025. PMID: 40488038 Free PMC article.
References
-
- Sinnwell M.A., Santana C.L., Bosch E., MacGillivray L.R., Groeneman R.H. Application of a tetrapyrimidyl cyclobutane synthesized in the organic solid state: A halogen-bonded supramolecular ladder. CrystEngComm. 2020;22:6780–6782. doi: 10.1039/D0CE01280G. - DOI
-
- Oburn S.M., Sinnwell M.A., Ericson D.P., Reinheimer E.W., Proserpio D.M., Groeneman R.H., MacGillivray L. Diversifying molecular and topological space via a supramolecular solid-state synthesis: A purely organic mok net sustained by hydrogen bonds. IUCrJ. 2019;6:1032–1039. doi: 10.1107/S2052252519011382. - DOI - PMC - PubMed
-
- Oburn S.M., Santana C.L., Elacqua E., Groeneman R.H. A diamondoid net sustained by halogen bonds: Employing a cyclobutane to generate a tetrahedral architecture. CrystEngComm. 2020;22:4349–4352. doi: 10.1039/D0CE00627K. - DOI
-
- Cohen M.D., Schmidt G.M.J. Topochemistry. Part I. A Survey. J. Chem. Soc. 1964:1996–2000. doi: 10.1039/jr9640001996. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

