The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules
- PMID: 35164339
- PMCID: PMC8839730
- DOI: 10.3390/molecules27031075
The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules
Abstract
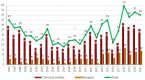
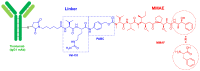
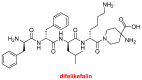
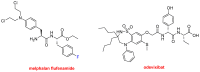
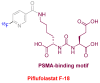
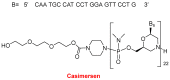
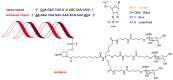
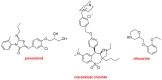
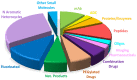
Similar to last year, 2021 will be remembered for the COVID-19 pandemic. Although five vaccines have been approved by the two most important drug regulatory agencies, namely the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), the pandemic has still not been brought under control. However, despite the context of a global pandemic, 2021 has been an excellent year with respect to drug approvals by the FDA. In 2021, 50 drugs have been authorized, making it the fourth-best year after 2018 (59 drugs) and 1996 and 2020 (53 each). Regarding biologics, 2021 has been the third-best year to date, with 14 approvals, and it has also witnessed the authorization of 36 small molecules. Of note, nine peptides, eight monoclonal antibodies, two antibody-drug conjugates, and two oligonucleotides have been approved this year. From them, five of the molecules are pegylated and three of them highly pegylated. The presence of nitrogen aromatic heterocycles and/or fluorine atoms are once again predominant among the so-called small molecules. This report analyzes the 50 new drugs approved in 2021 from a chemical perspective, as it did for those authorized in the previous five years. On the basis of chemical structure alone, the drugs that received approval in 2021 are classified as the following: biologics (antibodies, antibody-drug conjugates, enzymes, and pegylated proteins); TIDES (peptide and oligonucleotides); combined drugs; natural products; nitrogen aromatic heterocycles; fluorine-containing molecules; and other small molecules.
Keywords: API; CBER; CDER; COVID-19; TIDES; antibodies; antibody-drug conjugate; biologics; chemical entities; drug discovery; fluorine-based drugs; natural products; nitrogen aromatic heterocycles; oligonucleotides; pegylation; peptides; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



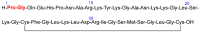

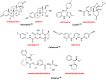
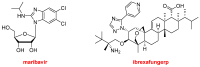
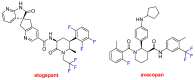
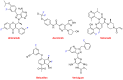

References
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism....
-
- European Medicines Agency (EMA) [(accessed on 1 January 2021)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-c....
-
- European Medicines Agency (EMA) [(accessed on 1 January 2021)]. Available online: https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu.
-
- U.S. Food and Drug Administration (FDA) [(accessed on 1 January 2021)]; Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

