Flavonoids: Antiplatelet Effect as Inhibitors of COX-1
- PMID: 35164411
- PMCID: PMC8839657
- DOI: 10.3390/molecules27031146
Flavonoids: Antiplatelet Effect as Inhibitors of COX-1
Abstract
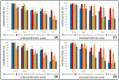
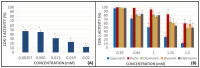
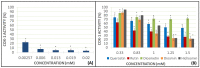
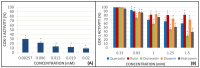
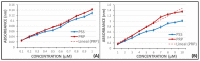
Flavonoids are compounds with a benzopyranic structure that exhibits multiple pharmacological activities. They are known for their venotonic activity, but their mechanism of action remains unclear. It is thought that, as this mechanism is mediated by prostaglandins, these compounds may interfere with the arachidonic acid (AA) cascade. These assays are designed to measure the antiplatelet aggregation capacity of quercetin, rutin, diosmetin, diosmin, and hidrosmin, as well as to evaluate a potential structure-activity ratio. In this paper, several studies on platelet aggregation at different concentrations (from 0.33 mM to 1.5 mM) of different flavone compounds are conducted, measuring platelet aggregation by impedance aggregometry, and the cyclooxygenase (COX) activity by metabolites generated, including the activity of the pure recombinant enzyme in the presence of these polyphenols. The results obtained showed that quercetin and diosmetin aglycones have a greater antiplatelet effect and inhibit the COX enzyme activity to a greater extent than their heterosides; however, the fact that greater inhibition of the pure recombinant enzyme was achieved by heterosides suggests that these compounds may have difficulty in crossing biological membranes. In any case, in view of the results obtained, it can be concluded that flavonoids could be useful as coadjuvants in the treatment of cardiovascular pathologies.
Keywords: antiplatelet activity; arachidonic acid (AA); cyclooxygenase (COX); flavonoids; impedance aggregometry; malondialdehyde (MDA); thromboxane B2 (TXB2).
Conflict of interest statement
The authors declare there is no conflict of interest whatsoever. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Lietti A., Cristoni A., Picci M. Studies on Vaccinium Myrtillus Anthocyanosides. I. Vasoprotective and Antiinflammatory Activity. Arzneimittelforschung. 1976;26:829–832. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

