Comparison of Large Animal Models for Acute Ischemic Stroke: Which Model to Use?
- PMID: 35164533
- PMCID: PMC10962757
- DOI: 10.1161/STROKEAHA.121.036050
Comparison of Large Animal Models for Acute Ischemic Stroke: Which Model to Use?
Abstract
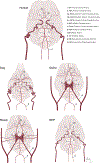
Translation of acute ischemic stroke research to the clinical setting remains limited over the last few decades with only one drug, recombinant tissue-type plasminogen activator, successfully completing the path from experimental study to clinical practice. To improve the selection of experimental treatments before testing in clinical studies, the use of large gyrencephalic animal models of acute ischemic stroke has been recommended. Currently, these models include, among others, dogs, swine, sheep, and nonhuman primates that closely emulate aspects of the human setting of brain ischemia and reperfusion. Species-specific characteristics, such as the cerebrovascular architecture or pathophysiology of thrombotic/ischemic processes, significantly influence the suitability of a model to address specific research questions. In this article, we review key characteristics of the main large animal models used in translational studies of acute ischemic stroke, regarding (1) anatomy and physiology of the cerebral vasculature, including brain morphology, coagulation characteristics, and immune function; (2) ischemic stroke modeling, including vessel occlusion approaches, reproducibility of infarct size, procedural complications, and functional outcome assessment; and (3) implementation aspects, including ethics, logistics, and costs. This review specifically aims to facilitate the selection of the appropriate large animal model for studies on acute ischemic stroke, based on specific research questions and large animal model characteristics.
Keywords: brain ischemia; dogs; models, animal; sheep; stroke; swine.
Figures
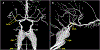

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

