Carbon Catabolite Repression Governs Diverse Physiological Processes and Development in Aspergillus nidulans
- PMID: 35164551
- PMCID: PMC8844935
- DOI: 10.1128/mbio.03734-21
Carbon Catabolite Repression Governs Diverse Physiological Processes and Development in Aspergillus nidulans
Abstract
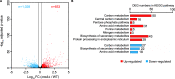
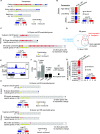
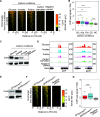
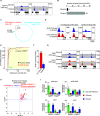
Carbon catabolite repression (CCR) is a common phenomenon of microorganisms that enable efficient utilization of carbon nutrients, critical for the fitness of microorganisms in the wild and for pathogenic species to cause infection. In most filamentous fungal species, the conserved transcription factor CreA/Cre1 mediates CCR. Previous studies demonstrated a primary function for CreA/Cre1 in carbon metabolism; however, the phenotype of creA/cre1 mutants indicated broader roles. The global function and regulatory mechanism of this wide-domain transcription factor has remained elusive. Here, we applied two powerful genomics methods (transcriptome sequencing and chromatin immunoprecipitation sequencing) to delineate the direct and indirect roles of Aspergillus nidulans CreA across diverse physiological processes, including secondary metabolism, iron homeostasis, oxidative stress response, development, N-glycan biosynthesis, unfolded protein response, and nutrient and ion transport. The results indicate intricate connections between the regulation of carbon metabolism and diverse cellular functions. Moreover, our work also provides key mechanistic insights into CreA regulation and identifies CreA as a master regulator controlling many transcription factors of different regulatory networks. The discoveries for this highly conserved transcriptional regulator in a model fungus have important implications for CCR in related pathogenic and industrial species. IMPORTANCE The ability to scavenge and use a wide range of nutrients for growth is crucial for microorganisms' survival in the wild. Carbon catabolite repression (CCR) is a transcriptional regulatory phenomenon of both bacteria and fungi to coordinate the expression of genes required for preferential utilization of carbon sources. Since carbon metabolism is essential for growth, CCR is central to the fitness of microorganisms. In filamentous fungi, CCR is mediated by the conserved transcription factor CreA/Cre1, whose function in carbon metabolism has been well established. However, the global roles and regulatory mechanism of CreA/Cre1 are poorly defined. This study uncovers the direct and indirect functions of CreA in the model organism Aspergillus nidulans over diverse physiological processes and development and provides mechanistic insights into how CreA controls different regulatory networks. The work also reveals an interesting functional divergence between filamentous fungal and yeast CreA/Cre1 orthologues.
Keywords: carbon catabolite repression; carbon metabolism; fungal physiology; gene regulation; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Regulation of Aspergillus nidulans CreA-Mediated Catabolite Repression by the F-Box Proteins Fbx23 and Fbx47.mBio. 2018 Jun 19;9(3):e00840-18. doi: 10.1128/mBio.00840-18. mBio. 2018. PMID: 29921666 Free PMC article.
-
Carbon Catabolite Repression in Filamentous Fungi Is Regulated by Phosphorylation of the Transcription Factor CreA.mBio. 2021 Jan 5;12(1):e03146-20. doi: 10.1128/mBio.03146-20. mBio. 2021. PMID: 33402538 Free PMC article.
-
Diverse Regulation of the CreA Carbon Catabolite Repressor in Aspergillus nidulans.Genetics. 2016 May;203(1):335-52. doi: 10.1534/genetics.116.187872. Epub 2016 Mar 26. Genetics. 2016. PMID: 27017621 Free PMC article.
-
Carbon Catabolite Repression in Filamentous Fungi.Int J Mol Sci. 2017 Dec 24;19(1):48. doi: 10.3390/ijms19010048. Int J Mol Sci. 2017. PMID: 29295552 Free PMC article. Review.
-
Implications of carbon catabolite repression for Aspergillus-based cell factories: A review.Biotechnol J. 2024 Feb;19(2):e2300551. doi: 10.1002/biot.202300551. Biotechnol J. 2024. PMID: 38403447 Review.
Cited by
-
Combined Transcriptome and Metabolome Analysis Reveals That Carbon Catabolite Repression Governs Growth and Pathogenicity in Verticillium dahliae.Int J Mol Sci. 2024 Oct 28;25(21):11575. doi: 10.3390/ijms252111575. Int J Mol Sci. 2024. PMID: 39519126 Free PMC article.
-
Advances in fungal sugar transporters: unlocking the potential of second-generation bioethanol production.Appl Microbiol Biotechnol. 2025 Jan 22;109(1):19. doi: 10.1007/s00253-025-13408-2. Appl Microbiol Biotechnol. 2025. PMID: 39841260 Free PMC article. Review.
-
Glucose Catabolite Repression Participates in the Regulation of Sialidase Biosynthesis by Antarctic Strain Penicillium griseofulvum P29.J Fungi (Basel). 2024 Mar 23;10(4):241. doi: 10.3390/jof10040241. J Fungi (Basel). 2024. PMID: 38667912 Free PMC article.
-
Biosynthesis of bromoform by Curvularia fungi provides a natural pathway to mitigate enteric methane emissions from ruminants.Biotechnol Rep (Amst). 2025 Jan 14;45:e00876. doi: 10.1016/j.btre.2025.e00876. eCollection 2025 Mar. Biotechnol Rep (Amst). 2025. PMID: 39906407 Free PMC article.
-
Regulation of nutrient utilization in filamentous fungi.Appl Microbiol Biotechnol. 2023 Oct;107(19):5873-5898. doi: 10.1007/s00253-023-12680-4. Epub 2023 Aug 4. Appl Microbiol Biotechnol. 2023. PMID: 37540250 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
