Evolutionary Sweeps of Subviral Parasites and Their Phage Host Bring Unique Parasite Variants and Disappearance of a Phage CRISPR-Cas System
- PMID: 35164562
- PMCID: PMC8844924
- DOI: 10.1128/mbio.03088-21
Evolutionary Sweeps of Subviral Parasites and Their Phage Host Bring Unique Parasite Variants and Disappearance of a Phage CRISPR-Cas System
Abstract
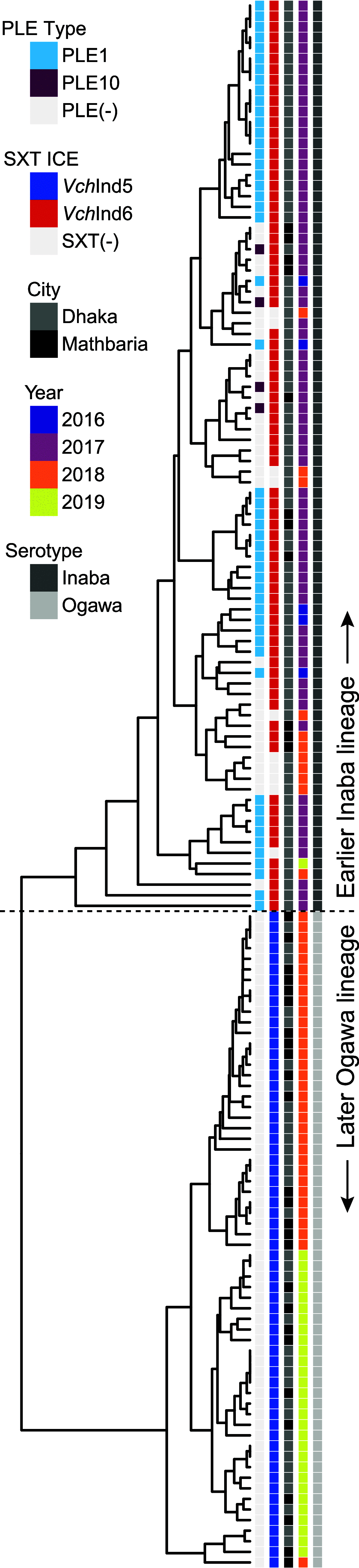
Vibrio cholerae is a significant threat to global public health in part due to its propensity for large-scale evolutionary sweeps where lineages emerge and are replaced. These sweeps may originate from the Bay of Bengal, where bacteriophage predation and the evolution of antiphage counterdefenses is a recurring theme. The bacteriophage ICP1 is a key predator of epidemic V. cholerae and is notable for acquiring a CRISPR-Cas system to combat PLE, a defensive subviral parasite encoded by its V. cholerae host. Here, we describe the discovery of four previously unknown PLE variants through a retrospective analysis of >3,000 publicly available sequences as well as one additional variant (PLE10) from recent surveillance of cholera patients in Bangladesh. In recent sampling we also observed a lineage sweep of PLE-negative V. cholerae occurring within the patient population in under a year. This shift coincided with a loss of ICP1's CRISPR-Cas system in favor of a previously prevalent PLE-targeting endonuclease called Odn. Interestingly, PLE10 was resistant to ICP1-encoded Odn, yet it was not found in any recent V. cholerae strains. We also identified isolates from within individual patient samples that revealed both mixed PLE(+)/PLE(-) V. cholerae populations and ICP1 strains possessing CRISPR-Cas or Odn with evidence of in situ recombination. These findings reinforce our understanding of the successive nature of V. cholerae evolution and suggest that ongoing surveillance of V. cholerae, ICP1, and PLE in Bangladesh is important for tracking genetic developments relevant to pandemic cholera that can occur over relatively short timescales. IMPORTANCE With 1 to 4 million estimated cases annually, cholera is a disease of serious global concern in regions where access to safe drinking water is limited by inadequate infrastructure, inequity, or natural disaster. The Global Task Force on Cholera Control (GTFCC.org) considers outbreak surveillance to be a primary pillar in the strategy to reduce mortality from cholera worldwide. Therefore, developing a better understanding of temporal evolutionary changes in the causative agent of cholera, Vibrio cholerae, could help in those efforts. The significance of our research is in tracking the genomic shifts that distinguish V. cholerae outbreaks, with specific attention paid to current and historical trends in the arms race between V. cholerae and a cooccurring viral (bacteriophage) predator. Here, we discover additional diversity of a specific phage defense system in epidemic V. cholerae and document the loss of a phage-encoded CRISPR-Cas system, underscoring the dynamic nature of microbial populations across cholera outbreaks.
Keywords: CRISPR-Cas; Vibrio cholerae; bacteriophages; cholera; evolution.
Conflict of interest statement
The authors declare a conflict of interest. K.D.S. is a scientific adviser for Nextbiotics Inc. All other authors declare no competing interests. The project described was supported by Grant Numbers R01AI127652 and R01AI153303 (K.D.S.) from the National Institute of Allergy and Infectious Diseases and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or NIH. K.D.S. is a Chan Zuckerberg Biohub Investigator and holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. icddr,b gratefully acknowledges the following donors, which provide unrestricted support: Government of the People's Republic of Bangladesh, Global Affairs Canada, Swedish International Development Cooperation Agency (SIDA), and the Department for International Development, UK Aid.
Figures

References
-
- Weill F-X, Domman D, Njamkepo E, Tarr C, Rauzier J, Fawal N, Keddy KH, Salje H, Moore S, Mukhopadhyay AK, Bercion R, Luquero FJ, Ngandjio A, Dosso M, Monakhova E, Garin B, Bouchier C, Pazzani C, Mutreja A, Grunow R, Sidikou F, Bonte L, Breurec S, Damian M, Njanpop-Lafourcade B-M, Sapriel G, Page A-L, Hamze M, Henkens M, Chowdhury G, Mengel M, Koeck J-L, Fournier J-M, Dougan G, Grimont PAD, Parkhill J, Holt KE, Piarroux R, Ramamurthy T, Quilici M-L, Thomson NR. 2017. Genomic history of the seventh pandemic of cholera in Africa. Science 358:785–789. doi:10.1126/science.aad5901. - DOI - PubMed
