Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection
- PMID: 35164565
- PMCID: PMC8942462
- DOI: 10.1128/mbio.03873-21
Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection
Abstract
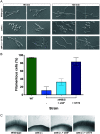
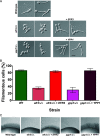
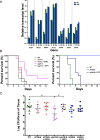
Phosphatidylinositol phosphates are key phospholipids with a range of regulatory roles, including membrane trafficking and cell polarity. Phosphatidylinositol-4-phosphate [PI(4)P] at the Golgi apparatus is required for the budding-to-filamentous-growth transition in the human-pathogenic fungus Candida albicans; however, the role of plasma membrane PI(4)P is unclear. We have investigated the importance of this phospholipid in C. albicans growth, stress response, and virulence by generating mutant strains with decreased levels of plasma membrane PI(4)P, via deletion of components of the PI-4-kinase complex, i.e., Efr3, Ypp1, and Stt4. The amounts of plasma membrane PI(4)P in the efr3Δ/Δ and ypp1Δ/Δ mutants were ∼60% and ∼40%, respectively, of that in the wild-type strain, whereas it was nearly undetectable in the stt4Δ/Δ mutant. All three mutants had reduced plas7ma membrane phosphatidylserine (PS). Although these mutants had normal yeast-phase growth, they were defective in filamentous growth, exhibited defects in cell wall integrity, and had an increased exposure of cell wall β(1,3)-glucan, yet they induced a range of hyphal-specific genes. In a mouse model of hematogenously disseminated candidiasis, fungal plasma membrane PI(4)P levels directly correlated with virulence; the efr3Δ/Δ mutant had wild-type virulence, the ypp1Δ/Δ mutant had attenuated virulence, and the stt4Δ/Δ mutant caused no lethality. In the mouse model of oropharyngeal candidiasis, only the ypp1Δ/Δ mutant had reduced virulence, indicating that plasma membrane PI(4)P is less important for proliferation in the oropharynx. Collectively, these results demonstrate that plasma membrane PI(4)P levels play a central role in filamentation, cell wall integrity, and virulence in C. albicans. IMPORTANCE While the PI-4-kinases Pik1 and Stt4 both produce PI(4)P, the former generates PI(4)P at the Golgi apparatus and the latter at the plasma membrane, and these two pools are functionally distinct. To address the importance of plasma membrane PI(4)P in Candida albicans, we generated deletion mutants of the three putative plasma membrane PI-4-kinase complex components and quantified the levels of plasma membrane PI(4)P in each of these strains. Our work reveals that this phosphatidylinositol phosphate is specifically critical for the yeast-to-hyphal transition, cell wall integrity, and virulence in a mouse systemic infection model. The significance of this work is in identifying a plasma membrane phospholipid that has an infection-specific role, which is attributed to the loss of plasma membrane PI(4)P resulting in β(1,3)-glucan unmasking.
Keywords: Candida albicans; cell wall; filamentous growth; opportunistic fungi; phosphatidylinositol phosphates; phospholipids; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Plasma Membrane Phosphatidylinositol 4-Phosphate Is Necessary for Virulence of Candida albicans.mBio. 2022 Jun 28;13(3):e0036622. doi: 10.1128/mbio.00366-22. Epub 2022 Apr 25. mBio. 2022. PMID: 35467420 Free PMC article.
-
The Candida albicans phosphatase Inp51p interacts with the EH domain protein Irs4p, regulates phosphatidylinositol-4,5-bisphosphate levels and influences hyphal formation, the cell integrity pathway and virulence.Microbiology (Reading). 2008 Nov;154(Pt 11):3296-3308. doi: 10.1099/mic.0.2008/018002-0. Microbiology (Reading). 2008. PMID: 18957583
-
Profilin Pfy1 is critical for cell wall integrity and virulence in Candida albicans.Microbiol Spectr. 2025 Apr;13(4):e0259324. doi: 10.1128/spectrum.02593-24. Epub 2025 Feb 24. Microbiol Spectr. 2025. PMID: 39992147 Free PMC article.
-
Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans.Microbiol Res. 2022 May;258:126996. doi: 10.1016/j.micres.2022.126996. Epub 2022 Feb 22. Microbiol Res. 2022. PMID: 35247799 Review.
-
Candida albicans the main opportunistic pathogenic fungus in humans.Rev Argent Microbiol. 2023 Apr-Jun;55(2):189-198. doi: 10.1016/j.ram.2022.08.003. Epub 2022 Nov 18. Rev Argent Microbiol. 2023. PMID: 36411138 Review.
Cited by
-
Regulation of yeast polarized exocytosis by phosphoinositide lipids.Cell Mol Life Sci. 2024 Nov 19;81(1):457. doi: 10.1007/s00018-024-05483-x. Cell Mol Life Sci. 2024. PMID: 39560727 Free PMC article. Review.
-
EFR3A, an Intriguing Gene, and Protein with a Scaffolding Function.Cells. 2025 Mar 17;14(6):445. doi: 10.3390/cells14060445. Cells. 2025. PMID: 40136694 Free PMC article. Review.
-
Two distinct lipid transporters together regulate invasive filamentous growth in the human fungal pathogen Candida albicans.PLoS Genet. 2022 Dec 14;18(12):e1010549. doi: 10.1371/journal.pgen.1010549. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36516161 Free PMC article.
-
Plasma Membrane Phosphatidylinositol 4-Phosphate Is Necessary for Virulence of Candida albicans.mBio. 2022 Jun 28;13(3):e0036622. doi: 10.1128/mbio.00366-22. Epub 2022 Apr 25. mBio. 2022. PMID: 35467420 Free PMC article.
-
The IV International Symposium on Fungal Stress and the XIII International Fungal Biology Conference.Fungal Biol. 2023 Jul-Aug;127(7-8):1157-1179. doi: 10.1016/j.funbio.2023.04.006. Epub 2023 May 11. Fungal Biol. 2023. PMID: 37495306 Free PMC article.
References
-
- Lee KT, So YS, Yang DH, Jung KW, Choi J, Lee DG, Kwon H, Jang J, Wang LL, Cha S, Meyers GL, Jeong E, Jin JH, Lee Y, Hong J, Bang S, Ji JH, Park G, Byun HJ, Park SW, Park YM, Adedoyin G, Kim T, Averette AF, Choi JS, Heitman J, Cheong E, Lee YH, Bahn YS. 2016. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans. Nat Commun 7:12766. doi:10.1038/ncomms12766. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
