Monoclonal antibody therapies in the management of SARS-CoV-2 infection
- PMID: 35164631
- PMCID: PMC8862171
- DOI: 10.1080/13543784.2022.2030310
Monoclonal antibody therapies in the management of SARS-CoV-2 infection
Abstract
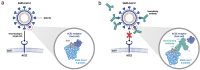
Introduction: Neutralizing antibodies (NAbs) that target key domains of the spike protein in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may have therapeutic value because of their specificity. Depending on the targeted epitope, single agents may be effective, but combined treatment involving multiple NAbs may be necessary to prevent the emergence of resistant variants.
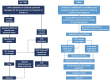
Areas covered: This article highlights the accelerated regulatory processes established to facilitate the review and approval of potential therapies. An overview of treatment approaches for SARS-CoV-2 infection, with detailed examination of the preclinical and clinical evidence supporting the use of NAbs, is provided. Finally, insights are offered into the potential benefits and challenges associated with the use of these agents.
Expert opinion: NAbs offer an effective, evidence-based therapeutic intervention during the early stages of SARS-CoV-2 infection when viral replication is the primary factor driving disease progression. As the pandemic progresses, appropriate use of NAbs will be important to minimize the risk of escape variants. Ultimately, the availability of effective treatments for COVID-19 will allow the establishment of treatment algorithms for minimizing the substantial rates of hospitalization, morbidity (including long COVID) and mortality currently associated with the disease.
Keywords: Bamlanivimab and etesevimab; COVID-19; SARS-CoV-2; casirivimab and imdevimab; neutralizing antibodies; regdanvimab; sotrovimab.
Figures
Similar articles
-
Comparison of Dual Monoclonal Antibody Therapies for COVID-19 Evolution: A Multicentric Retrospective Study.Viruses. 2024 Sep 29;16(10):1542. doi: 10.3390/v16101542. Viruses. 2024. PMID: 39459877 Free PMC article.
-
Neutralizing Antibody Therapeutics for COVID-19.Viruses. 2021 Apr 7;13(4):628. doi: 10.3390/v13040628. Viruses. 2021. PMID: 33916927 Free PMC article. Review.
-
Curbing the Delta Surge: Clinical Outcomes After Treatment With Bamlanivimab-Etesevimab, Casirivimab-Imdevimab, or Sotrovimab for Mild to Moderate Coronavirus Disease 2019.Mayo Clin Proc. 2022 Sep;97(9):1641-1648. doi: 10.1016/j.mayocp.2022.06.015. Epub 2022 Jun 23. Mayo Clin Proc. 2022. PMID: 36058578 Free PMC article.
-
Neutralizing anti-spike monoclonal antibodies for COVID-19 in vulnerable populations: lessons learned and future directions.Expert Opin Biol Ther. 2023 Jul-Dec;23(7):619-631. doi: 10.1080/14712598.2023.2226326. Epub 2023 Jun 18. Expert Opin Biol Ther. 2023. PMID: 37318043 Review.
-
Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern.Elife. 2022 Nov 22;11:e79639. doi: 10.7554/eLife.79639. Elife. 2022. PMID: 36413383 Free PMC article. Clinical Trial.
Cited by
-
Population pharmacokinetics and exposure-response analysis of a single dose of sotrovimab in the early treatment of patients with mild to moderate COVID-19.CPT Pharmacometrics Syst Pharmacol. 2023 Jun;12(6):853-864. doi: 10.1002/psp4.12958. Epub 2023 Apr 6. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36922886 Free PMC article.
-
Use of the Monoclonal Antibody Regdanvimab to Treat Patients Hospitalized with COVID-19: Real-World Data during the Delta Variant Predominance.Infect Chemother. 2022 Dec;54(4):781-786. doi: 10.3947/ic.2022.0103. Epub 2022 Sep 7. Infect Chemother. 2022. PMID: 36226346 Free PMC article.
-
Nanoscale warriors against viral invaders: a comprehensive review of Nanobodies as potential antiviral therapeutics.MAbs. 2025 Dec;17(1):2486390. doi: 10.1080/19420862.2025.2486390. Epub 2025 Apr 9. MAbs. 2025. PMID: 40201976 Free PMC article. Review.
-
T cells in SARS-CoV-2 infection and vaccination.Ther Adv Vaccines Immunother. 2022 Aug 24;10:25151355221115011. doi: 10.1177/25151355221115011. eCollection 2022. Ther Adv Vaccines Immunother. 2022. PMID: 36051003 Free PMC article. Review.
-
Synthesis of conjugates of (aR,7S)-colchicine with monoterpenoids and investigation of their biological activity.Russ Chem Bull. 2023;72(1):248-262. doi: 10.1007/s11172-023-3730-4. Epub 2023 Feb 14. Russ Chem Bull. 2023. PMID: 36817557 Free PMC article.
References
-
- World Health Organization . WHO Coronavirus (COVID-19) dashboard. 2021. [cited 2021 May 23]; Available from: https://covid19.who.int/
-
- Kim PS, Read SW, Fauci AS.. Therapy for early COVID-19: a critical need. JAMA. 2020;324(21):2149–2150. - PubMed
-
- World Health Organization . SARS-CoV-2 variants. 2020. [cited 2021 May 23]; Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON305
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
