Somatostatin attenuates intestinal epithelial barrier injury during acute intestinal ischemia-reperfusion through Tollip/Myeloiddifferentiationfactor 88/Nuclear factor kappa-B signaling
- PMID: 35164650
- PMCID: PMC8973595
- DOI: 10.1080/21655979.2022.2038450
Somatostatin attenuates intestinal epithelial barrier injury during acute intestinal ischemia-reperfusion through Tollip/Myeloiddifferentiationfactor 88/Nuclear factor kappa-B signaling
Abstract
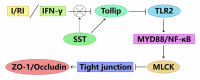
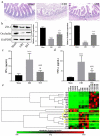
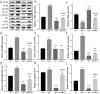
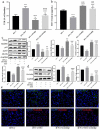
In the process of ischemia-reperfusion injury, intestinal ischemia and inflammation interweave, leading to tissue damage or necrosis. However, oxygen radicals and inflammatory mediators produced after reperfusion cause tissue damage again, resulting in severe intestinal epithelial barrier dysfunction. The aim of this study was to determine the protective effect of somatostatin on intestinal epithelial barrier function during intestinal ischemia-reperfusion injury and explore its mechanism. By establishing a rat intestinal ischemia-reperfusion model, pretreating the rats with somatostatin, and then detecting the histopathological changes, intestinal permeability and expression of tight junction proteins in intestinal tissues, the protective effect of somatostatin on the intestinal epithelial barrier was measured in vivo. The mechanism was determined in interferon γ (IFN-γ)-treated Caco-2 cells in vitro. The results showed that somatostatin could ameliorate ischemia-reperfusion-induced intestinal epithelial barrier dysfunction and protect Caco-2 cells against IFN-γ-induced decreases in tight junction protein expression and increases in monolayer cell permeability. The expression of Tollip was upregulated by somatostatin both in ischemia-reperfusion rats and IFN-γ-treated Caco-2 cells, while the activation of TLR2/MyD88/NF-κB signaling was inhibited by somatostatin. Tollip inhibition reversed the protective effect of somatostatin on the intestinal epithelial barrier. In conclusion, somatostatin could attenuate ischemia-reperfusion-induced intestinal epithelial barrier injury by inhibiting the activation of TLR2/MyD88/NF-κB signaling through upregulation of Tollip.
Keywords: Intestinal ischemia–reperfusion injury; Tollip; intestinal epithelial barrier; somatostatin; tight junction.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Dexmedetomidine Resists Intestinal Ischemia-Reperfusion Injury by Inhibiting TLR4/MyD88/NF-κB Signaling.J Surg Res. 2021 Apr;260:350-358. doi: 10.1016/j.jss.2020.11.041. Epub 2020 Dec 28. J Surg Res. 2021. PMID: 33383282
-
Amelioration of IFN-γ and TNF-α-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway.PLoS One. 2013 May 3;8(5):e61944. doi: 10.1371/journal.pone.0061944. Print 2013. PLoS One. 2013. PMID: 23671580 Free PMC article.
-
Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway.J Interferon Cytokine Res. 2014 Mar;34(3):195-203. doi: 10.1089/jir.2013.0044. Epub 2013 Nov 15. J Interferon Cytokine Res. 2014. PMID: 24237301
-
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 8;9(1):12. doi: 10.1038/s41392-023-01688-x. Signal Transduct Target Ther. 2024. PMID: 38185705 Free PMC article. Review.
-
Mechanism Involved in Acute Liver Injury Induced by Intestinal Ischemia-Reperfusion.Front Pharmacol. 2022 May 23;13:924695. doi: 10.3389/fphar.2022.924695. eCollection 2022. Front Pharmacol. 2022. PMID: 35694264 Free PMC article. Review.
Cited by
-
Intestinal Microbiota - An Unmissable Bridge to Severe Acute Pancreatitis-Associated Acute Lung Injury.Front Immunol. 2022 Jun 14;13:913178. doi: 10.3389/fimmu.2022.913178. eCollection 2022. Front Immunol. 2022. PMID: 35774796 Free PMC article. Review.
-
Somatostatin peptides prevent increased human colonic epithelial permeability induced by hypoxia.Am J Physiol Gastrointest Liver Physiol. 2024 Nov 1;327(5):G701-G710. doi: 10.1152/ajpgi.00057.2024. Epub 2024 Sep 3. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 39226584
-
Evaluation of Endoplasmic Reticulum Stress in an Experimental Intestinal Ischemia-Reperfusion Model in Rats: The Role of Ozone Therapy and Trimetazidine.Biomolecules. 2024 Aug 25;14(9):1051. doi: 10.3390/biom14091051. Biomolecules. 2024. PMID: 39334818 Free PMC article.
-
Somatostatin and N-acetylcysteine on testicular damage triggered by ischemia reperfusion: cellular protection and antioxidant effects.Hormones (Athens). 2025 Apr 12. doi: 10.1007/s42000-025-00650-6. Online ahead of print. Hormones (Athens). 2025. PMID: 40220169
-
Intestinal oxygen utilisation and cellular adaptation during intestinal ischaemia-reperfusion injury.Clin Transl Med. 2025 Jan;15(1):e70136. doi: 10.1002/ctm2.70136. Clin Transl Med. 2025. PMID: 39724463 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
