Extracellular microparticles derived from hepatic progenitor cells deliver a death signal to hepatoma-initiating cells
- PMID: 35164767
- PMCID: PMC8842981
- DOI: 10.1186/s12951-022-01280-5
Extracellular microparticles derived from hepatic progenitor cells deliver a death signal to hepatoma-initiating cells
Abstract
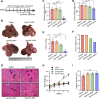
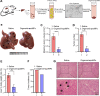
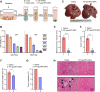
The malignant transformation of normal resident hepatic stem/progenitor cells has a critical role in hepatocarcinogenesis and the recurrence of hepatocellular carcinoma (HCC). We defined such hepatic progenitor cells as hepatoma-initiating cells. An efficient strategy is required to target and kill the hepatoma-initiating cells. We isolated extracellular microparticles (MPs) derived from apoptotic hepatic progenitor cells (HPCs) and tested their ability to inhibit hepatocarcinogenesis. Extracellular MPs were isolated from HPCs, hepatocytes and liver tumor cells. Their effects on tumor growth were investigated in rat primary HCC models, in which hepatocarcinogenesis is induced by diethylnitrosamine (DEN). The extracellular MPs derived from apoptotic HPCs, apoptotic hepatocytes and apoptotic liver tumor cells were similar in morphology, diameter and zeta potential. However, they had different antitumor effects. In DEN-exposed rats, only the MPs derived from apoptotic HPCs effectively inhibit hepatocarcinogenesis. In vitro and in vivo analyses confirmed that HPCs preferentially take up MPs derived from apoptotic HPCs compared to MPs from other liver cell types. Proteomic analysis of MPs from apoptotic HPCs showed enrichment of proteins involved in cell death pathways. Thus, HPC-derived MPs contain a death signal to induce the killing of hepatoma-initiating cells. Our findings provide evidence that a death signal encapsulated in HPC-derived extracellular microparticles can efficiently clear hepatoma-initiating cells and prevent hepatocarcinogenesis.
Keywords: Death signal; Extracellular microparticles; Hepatic progenitor cells; Hepatocarcinogenesis; Hepatoma-initiating cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

