Optimizing responsiveness to feedback about antibiotic prescribing in primary care: protocol for two interrelated randomized implementation trials with embedded process evaluations
- PMID: 35164805
- PMCID: PMC8842929
- DOI: 10.1186/s13012-022-01194-8
Optimizing responsiveness to feedback about antibiotic prescribing in primary care: protocol for two interrelated randomized implementation trials with embedded process evaluations
Abstract
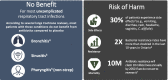
Background: Audit and feedback (A&F) that shows how health professionals compare to those of their peers, can be an effective intervention to reduce unnecessary antibiotic prescribing among family physicians. However, the most impactful design approach to A&F to achieve this aim is uncertain. We will test three design modifications of antibiotic A&F that could be readily scaled and sustained if shown to be effective: (1) inclusion of case-mix-adjusted peer comparator versus a crude comparator, (2) emphasizing harms, rather than lack of benefits, and (3) providing a viral prescription pad.
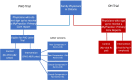
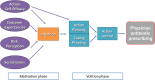
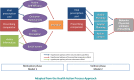
Methods: We will conduct two interrelated pragmatic randomized trials in January 2021. One trial will include family physicians in Ontario who have signed up to receive their MyPractice: Primary Care report from Ontario Health ("OH Trial"). These physicians will be cluster-randomized by practice, 1:1 to intervention or control. The intervention group will also receive a Viral Prescription Pad mailed to their office as well as added emphasis in their report on use of the pad. Ontario family physicians who have not signed up to receive their MyPractice: Primary Care report will be included in the other trial administered by Public Health Ontario ("PHO Trial"). These physicians will be allocated 4:1 to intervention or control. The intervention group will be further randomized by two factors: case-mix adjusted versus unadjusted comparator and emphasis or not on harms of antibiotics. Physicians in the intervention arm of this trial will receive one of four versions of a personalized antibiotic A&F letter from PHO. For both trials, the primary outcome is the antibiotic prescribing rate per 1000 patient visits, measured at 6 months post-randomization, the primary analysis will use Poisson regression and we will follow the intention to treat principle. A mixed-methods process evaluation will use surveys and interviews with family physicians to explore potential mechanisms underlying the observed effects, exploring targeted constructs including intention, self-efficacy, outcome expectancies, descriptive norms, and goal prioritization.
Discussion: This protocol describes the rationale and methodology of two interrelated pragmatic trials testing variations of theory-informed components of an audit and feedback intervention to determine how to optimize A&F interventions for antibiotic prescribing in primary care.
Trial registration: NCT04594200, NCT05044052. CIHR Grant ID: 398514.
Keywords: Antibiotic prescribing; Antimicrobial resistance; Audit and feedback; Process evaluation; Protocol.
© 2022. The Author(s).
Conflict of interest statement
JP is an associate editor of implementation science. JG and NMI are members of the editorial board of implementation science.
Figures



Similar articles
-
Mailed feedback to primary care physicians on antibiotic prescribing for patients aged 65 years and older: pragmatic, factorial randomised controlled trial.BMJ. 2024 Jun 5;385:e079329. doi: 10.1136/bmj-2024-079329. BMJ. 2024. PMID: 38839101 Free PMC article. Clinical Trial.
-
Process evaluation of two large randomized controlled trials to understand factors influencing family physicians' use of antibiotic audit and feedback reports.Implement Sci. 2024 Sep 16;19(1):65. doi: 10.1186/s13012-024-01393-5. Implement Sci. 2024. PMID: 39285305 Free PMC article. Clinical Trial.
-
Effect of Antibiotic Prescription Audit and Feedback on Antibiotic Prescribing in Primary Care: A Randomized Clinical Trial.JAMA Intern Med. 2023 Mar 1;183(3):213-220. doi: 10.1001/jamainternmed.2022.6529. JAMA Intern Med. 2023. PMID: 36745412 Free PMC article. Clinical Trial.
-
Audit and Feedback Interventions for Antibiotic Prescribing in Primary Care: A Systematic Review and Meta-analysis.Clin Infect Dis. 2025 Feb 24;80(2):253-262. doi: 10.1093/cid/ciae604. Clin Infect Dis. 2025. PMID: 39657007 Free PMC article.
-
Nudge interventions to reduce unnecessary antibiotic prescribing in primary care: a systematic review.BMJ Open. 2023 Jan 18;13(1):e062688. doi: 10.1136/bmjopen-2022-062688. BMJ Open. 2023. PMID: 36657758 Free PMC article.
Cited by
-
Mailed feedback to primary care physicians on antibiotic prescribing for patients aged 65 years and older: pragmatic, factorial randomised controlled trial.BMJ. 2024 Jun 5;385:e079329. doi: 10.1136/bmj-2024-079329. BMJ. 2024. PMID: 38839101 Free PMC article. Clinical Trial.
-
Spillover From an Intervention on Antibiotic Prescribing for Family Physicians: A Post Hoc Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2025 Jul 1;8(7):e2518261. doi: 10.1001/jamanetworkopen.2025.18261. JAMA Netw Open. 2025. PMID: 40591360 Free PMC article. Clinical Trial.
-
Moving the needle on dental antibiotic overuse in Canada post COVID-19.Can Commun Dis Rep. 2022 Nov 3;48(11-12):502-505. doi: 10.14745/ccdr.v48i1112a02. eCollection 2022 Nov 3. Can Commun Dis Rep. 2022. PMID: 38173695 Free PMC article.
-
Regional and national antimicrobial stewardship activities: a survey from the Joint Programming Initiative on Antimicrobial Resistance-Primary Care Antibiotic Audit and Feedback Network (JPIAMR-PAAN).JAC Antimicrob Resist. 2023 Apr 24;5(2):dlad048. doi: 10.1093/jacamr/dlad048. eCollection 2023 Apr. JAC Antimicrob Resist. 2023. PMID: 38659427 Free PMC article.
-
Process evaluation of two large randomized controlled trials to understand factors influencing family physicians' use of antibiotic audit and feedback reports.Implement Sci. 2024 Sep 16;19(1):65. doi: 10.1186/s13012-024-01393-5. Implement Sci. 2024. PMID: 39285305 Free PMC article. Clinical Trial.
References
-
- Schwartz KL, Brown KA, Etches J, Langford BJ, Daneman N, Tu K, Johnstone J, Achonu C, Garber G. Predictors and variability of antibiotic prescribing amongst family physicians. J Antimicrob Chemother. 2019;74(7):2098–2105. - PubMed
-
- Jones BE, Sauer B, Jones MM, Campo J, Damal K, He T, Ying J, Greene T, Goetz MB, Neuhauser MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med. 2015;163(2):73–80. - PubMed
-
- Public Health Agency of Canada . Canadian Antimicrobial Resistance Surveillance System report. Public Health Agency of Canada Ottawa, ON, Canada; 2020.
-
- Nudge vs Superbugs . a behavioural economics trial to reduce the overprescribing of antibiotics. Australian Government Department of Health; 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

