Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis
- PMID: 35164837
- PMCID: PMC8842872
- DOI: 10.1186/s13075-022-02738-5
Chondrocyte-derived exosomes promote cartilage calcification in temporomandibular joint osteoarthritis
Abstract
Backgrounds: Abnormal cartilage calcification is one of the pathological changes of temporomandibular joint (TMJ) osteoarthritis (OA). Recent studies have reported that exosomes can regulate the formation of abnormal calcified nodules in diseases including atherosclerosis and chronic kidney disease. However, the influences of chondrocyte-derived exosomes on abnormal cartilage calcification in TMJ OA are still unclear.
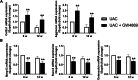
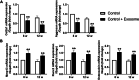
Methods: TMJ OA was induced by unilateral anterior crossbite (UAC) for 4, 8, or 12 weeks in rats to observe abnormal calcification in TMJ condylar cartilage and exosome formation. Concomitantly, GW4869, the inhibitor of exosome formation, was locally injected to the TMJ of rats under stimulation of UAC, while the exosomes extracted from primary condylar chondrocytes stimulated with fluid flow shear stress (FFSS) were locally injected to rats TMJ.
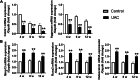
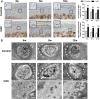
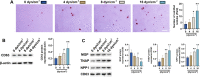
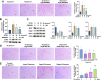
Results: Abnormal calcification was enhanced in the degenerative cartilage of TMJ OA in UAC rats, and a large number of exosome-like structures with diameters of 50-150 nm were found in the calcified cartilage together with decreased expression of matrix Gla protein (MGP) and increased expression of CD63, tissue-nonspecific alkaline phosphatase (TNAP) and nucleotide pyrophosphatase/phosphodiesterase-1 (NPP1). After FFSS stimulation, the number of exosomes secreted by chondrocytes and the numbers of calcified nodules were increased in cultured cells, and the protein levels of MGP, TNAP, and NPP1 in exosomes were changed. Inhibition of exosome formation, TNAP, and NPP1 or supplementation with exogenous MGP effectively alleviated FFSS-induced chondrocyte calcification. Local injection of GW4869, the exosome inhibitor, alleviated TMJ OA-related cartilage degeneration and calcification in UAC rats. Local injection of exosomes obtained from chondrocytes stimulated by FFSS to the TMJs of normal rats induced cartilage degeneration and calcification similar to that in TMJ OA.
Conclusions: Abnormal biomechanical loading leads to enhanced formation of chondrocyte-derived exosomes, in which promoters of calcification increased and inhibitors decreased, resulting in accelerating abnormal cartilage calcification in TMJ OA. The inhibition of degenerative chondrocyte-derived exosomes is expected to be a new way to prevent and treat TMJ OA.
Keywords: Cartilage calcification; Chondrocyte; Exosome; Osteoarthritis; Temporomandibular joint.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Decreased expression of ATP-binding cassette protein G1 promotes abnormal adipogenesis of condylar chondrocytes in temporomandibular joint osteoarthritis.J Oral Rehabil. 2024 May;51(5):805-816. doi: 10.1111/joor.13647. Epub 2023 Dec 26. J Oral Rehabil. 2024. PMID: 38146807
-
GDF11 inhibits abnormal adipogenesis of condylar chondrocytes in temporomandibular joint osteoarthritis.Bone Joint Res. 2022 Jul;11(7):453-464. doi: 10.1302/2046-3758.117.BJR-2022-0019.R1. Bone Joint Res. 2022. PMID: 35787089 Free PMC article.
-
MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint.Autophagy. 2020 Feb;16(2):271-288. doi: 10.1080/15548627.2019.1606647. Epub 2019 Apr 21. Autophagy. 2020. PMID: 31007149 Free PMC article.
-
Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint.J Cell Mol Med. 2021 Jun;25(11):4902-4911. doi: 10.1111/jcmm.16514. Epub 2021 May 5. J Cell Mol Med. 2021. PMID: 33949768 Free PMC article. Review.
-
Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis.Int J Mol Sci. 2020 Feb 24;21(4):1541. doi: 10.3390/ijms21041541. Int J Mol Sci. 2020. PMID: 32102392 Free PMC article. Review.
Cited by
-
A new frontier in temporomandibular joint osteoarthritis treatment: Exosome-based therapeutic strategy.Front Bioeng Biotechnol. 2022 Nov 25;10:1074536. doi: 10.3389/fbioe.2022.1074536. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36507254 Free PMC article. Review.
-
The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis.Cells. 2023 Nov 27;12(23):2716. doi: 10.3390/cells12232716. Cells. 2023. PMID: 38067147 Free PMC article. Review.
-
Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment.Front Cell Dev Biol. 2022 Jul 26;10:949690. doi: 10.3389/fcell.2022.949690. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35959489 Free PMC article. Review.
-
Exosomes in cartilage microenvironment regulation and cartilage repair.Front Cell Dev Biol. 2025 Mar 5;13:1460416. doi: 10.3389/fcell.2025.1460416. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40109360 Free PMC article. Review.
-
Microvesicles Released by Osteoclastic Cells Exhibited Chondrogenic, Osteogenic, and Anti-Inflammatory Activities: An Evaluation of the Feasibility of Their Use for Treatment of Osteoarthritis in a Mouse Model.Cells. 2025 Jan 28;14(3):193. doi: 10.3390/cells14030193. Cells. 2025. PMID: 39936984 Free PMC article.
References
-
- Zhao Y-p, Zhang Z-y, Wu Y-t, Zhang W-l, Ma X-c. Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(2):e27–e34. doi: 10.1016/j.tripleo.2010.09.076. - DOI - PubMed
-
- Kourí JB, Aguilera JM, Reyes J, Lozoya KA, González S. Apoptotic chondrocytes from osteoarthrotic human articular cartilage and abnormal calcification of subchondral bone. J Rheumatol. 2000;27(4):1005–1019. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

