Management of a human immunodeficiency virus case with discordant antiviral drug resistance profiles in cerebrospinal fluid compared with plasma: a case report
- PMID: 35164871
- PMCID: PMC8845297
- DOI: 10.1186/s13256-022-03289-8
Management of a human immunodeficiency virus case with discordant antiviral drug resistance profiles in cerebrospinal fluid compared with plasma: a case report
Abstract
Background: Human immunodeficiency virus-1-associated neurocognitive disorder is a known complication in individuals treated with antiretroviral therapy. Cerebrospinal fluid escape, which is defined as discordant higher cerebrospinal fluid viremia than plasma, may occur in antiretroviral therapy-experienced individuals. Different cerebrospinal fluid versus plasma mutation patterns have been observed in individuals with cerebrospinal fluid escape.
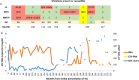
Case presentation: A 46-year-old adult African male with human immunodeficiency virus-1 infection and acquired immunodeficiency syndrome based on cerebral toxoplasmosis and a chronic hepatitis B virus infection developed cerebrospinal fluid escape. A different human immunodeficiency virus-1 genotypic drug resistance profile was observed in plasma compared with cerebrospinal fluid. Brain biopsy and cerebral magnetic resonance imaging indicated the development of human immunodeficiency virus encephalopathy. A discordant protease inhibitor mutation/wild-type T74PT in plasma but not in cerebrospinal fluid indicated poor central nervous system penetration due to the selective pressure of drug therapy. An intensified antiretroviral therapy regimen including dolutegravir with good central nervous system penetration improved conditions.
Conclusions: This case shows the importance of measuring human immunodeficiency virus drug resistance in cerebrospinal fluid, which might differ from resistance detected in plasma samples and target effective antiretroviral therapy treatment accordingly.
Keywords: AIDS; Cerebrospinal fluid; HIV; HIV-associated neurocognitive disorder; Protease inhibitor drug resistance.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Soulie C, Grudé M, Descamps D, Amiel C, Morand-Joubert L, Raymond S, et al. Antiretroviral-treated HIV-1 patients can harbour resistant viruses in CSF despite an undetectable viral load in plasma. J Antimicrob Chemother. 2017;72:2351–2354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

