A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: Safety, reactogenicity and immunogenicity
- PMID: 35164986
- PMCID: PMC8823954
- DOI: 10.1016/j.vaccine.2022.02.029
A randomized, double-blind phase I clinical trial of two recombinant dimeric RBD COVID-19 vaccine candidates: Safety, reactogenicity and immunogenicity
Abstract
Background: The Receptor Binding Domain (RBD) of the SARS-CoV-2 spike protein is the target for many COVID-19 vaccines. Here we report results for phase I clinical trial of two COVID-19 vaccine candidates based on recombinant dimeric RBD (d-RBD).
Methods: We performed a randomized, double-blind, phase I clinical trial in the National Centre of Toxicology in Havana. Sixty Cuban volunteers aged 19-59 years were randomized into three groups (20 subjects each): 1) FINLAY-FR-1 (50 µg d-RBD plus outer membrane vesicles from N. meningitidis); 2) FINLAY-FR-1A-50 (50 µg d-RBD, three doses); 3) FINLAY-FR-1A-25 (25 µg d-RDB, three doses). The FINLAY-FR-1 group was randomly divided to receive a third dose of the same vaccine candidate (homologous schedule) or FINLAY-FR-1A-50 (heterologous schedule). The primary outcomes were safety and reactogenicity. The secondary outcome was vaccine immunogenicity. Humoral response at baseline and following each vaccination was evaluated using live-virus neutralization test, anti-RBD IgG ELISA and in-vitro neutralization test of RBD:hACE2 interaction.
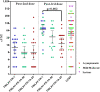
Results: Most adverse events were of mild intensity (63.5%), solicited (58.8%), and local (61.8%); 69.4% with causal association with vaccination. Serious adverse events were not found. The FINLAY-FR-1 group reported more subjects with adverse events than the other two groups. After the third dose, anti-RBD seroconversion was 100%, 94.4% and 90% for the FINLAY-FR-1, FINLAY-FR-1A-50 and FINLAY-FR-1A-25 respectively. The in-vitro inhibition of RBD:hACE2 interaction increased after the second dose in all formulations. The geometric mean neutralizing titres after the third dose rose significantly in the group vaccinated with FINLAY-FR-1 with respect to the other formulations and the COVID-19 Convalescent Serum Panel. No differences were found between FINLAY-FR-1 homologous or heterologous schedules.
Conclusions: Vaccine candidates were safe and immunogenic, and induced live-virus neutralizing antibodies against SARS-CoV-2. The highest values were obtained when outer membrane vesicles were used as adjuvant.
Trial registry: https://rpcec.sld.cu/en/trials/RPCEC00000338-En.
Keywords: Adjuvants; COVID-19; Coronavirus infection; Immunization schedule; Immunopotentiator; Neutralizing antibodies; SARS-CoV-2; Vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The Finlay Vaccine Institute and the Centre of Molecular Immunology have filed patent applications related to these vaccine candidates. ROA, MCRG, BPM, YCR, LRN, RPN, RGM, MMP, YVB, DGR, and VVB are researchers of Finlay Vaccine Institute, and THG, GBB, FPE and BSR are researchers of the Centre of Molecular Immunology, the institutions that manufacture the vaccines. The other authors declare no competing interests. No authors received an honorarium for this paper.
Figures
References
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Geneva: WHO; 2021. Available from: https://covid19.who.int.
-
- Shi-Lee W., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5:1185–1191. - PubMed
-
- Arvin A.M., Fink K., Schmid M.A., Cathcart A., Spreafico R., Havenar-Daughton C., et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821):353–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

