Lung directed antibody gene transfer confers protection against SARS-CoV-2 infection
- PMID: 35165144
- PMCID: PMC8861887
- DOI: 10.1136/thoraxjnl-2021-217650
Lung directed antibody gene transfer confers protection against SARS-CoV-2 infection
Abstract
Background: The COVID-19 pandemic continues to be a worldwide threat and effective antiviral drugs and vaccines are being developed in a joint global effort. However, some elderly and immune-compromised populations are unable to raise an effective immune response against traditional vaccines.
Aims: We hypothesised that passive immunity engineered by the in vivo expression of anti-SARS-CoV-2 monoclonal antibodies (mAbs), an approach termed vectored-immunoprophylaxis (VIP), could offer sustained protection against COVID-19 in all populations irrespective of their immune status or age.
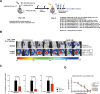
Methods: We developed three key reagents to evaluate VIP for SARS-CoV-2: (i) we engineered standard laboratory mice to express human ACE2 via rAAV9 in vivo gene transfer, to allow in vivo assessment of SARS-CoV-2 infection, (ii) to simplify in vivo challenge studies, we generated SARS-CoV-2 Spike protein pseudotyped lentiviral vectors as a simple mimic of authentic SARS-CoV-2 that could be used under standard laboratory containment conditions and (iii) we developed in vivo gene transfer vectors to express anti-SARS-CoV-2 mAbs.
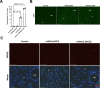
Conclusions: A single intranasal dose of rAAV9 or rSIV.F/HN vectors expressing anti-SARS-CoV-2 mAbs significantly reduced SARS-CoV-2 mimic infection in the lower respiratory tract of hACE2-expressing mice. If translated, the VIP approach could potentially offer a highly effective, long-term protection against COVID-19 for highly vulnerable populations; especially immune-deficient/senescent individuals, who fail to respond to conventional SARS-CoV-2 vaccines. The in vivo expression of multiple anti-SARS-CoV-2 mAbs could enhance protection and prevent rapid mutational escape.
Keywords: COVID-19; respiratory infection; viral infection.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: YD, KMM, DRG and SCH hold IP in relation to S-LV technology. DRG and SCH hold IP in relation to rSIV.F/HN technology.
Figures



References
-
- Ramasamy MN, Minassian AM, Ewer KJ, et al. . Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396:1979–93. 10.1016/S0140-6736(20)32466-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
