Nicotinic Acetylcholine Receptors Expressed by Striatal Interneurons Inhibit Striatal Activity and Control Striatal-Dependent Behaviors
- PMID: 35165173
- PMCID: PMC8973422
- DOI: 10.1523/JNEUROSCI.1627-21.2022
Nicotinic Acetylcholine Receptors Expressed by Striatal Interneurons Inhibit Striatal Activity and Control Striatal-Dependent Behaviors
Abstract
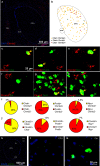
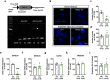
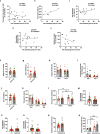
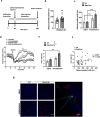
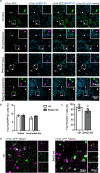
Acetylcholine is an important modulator of striatal activity, and it is vital to controlling striatal-dependent behaviors, including motor and cognitive functions. Despite this significance, the mechanisms determining how acetylcholine impacts striatal signaling are still not fully understood. In particular, little is known about the role of nAChRs expressed by striatal interneurons. In the present study, we used FISH to determine which neuronal types express the most prevalent beta2 nicotinic subunit in the mouse striatum. Our data support a common view that nAChR expression is mostly restricted to striatal interneurons. Surprisingly though, cholinergic interneurons were identified as a population with the highest expression of beta2 nicotinic subunit. To investigate the functional significance of beta2-containing nAChRs in striatal interneurons, we deleted them by injecting the AAV-Cre vector into the striatum of beta2-flox/flox male mice. The deletion led to alterations in several behavioral domains, namely, to an increased anxiety-like behavior, decrease in sociability ratio, deficit in discrimination learning, and increased amphetamine-induced hyperlocomotion and c-Fos expression in mice with beta2 deletion. Further colocalization analysis showed that the increased c-Fos expression was present in both medium spiny neurons and presumed striatal interneurons. The present study concludes that, despite being relatively rare, beta2-containing nAChRs are primarily expressed in striatal neurons by cholinergic interneurons and play a significant role in behavior.SIGNIFICANCE STATEMENT A large variety of nAChRs are expressed in the striatum, a brain region that is crucial in the control of behavior. The complexity of receptors with different functions is hindering our understanding of mechanisms through which striatal acetylcholine modulates behavior. We focused on the role of a small population of beta2-containing nAChRs. We identified neuronal types expressing these receptors and determined their impact in the control of explorative behavior, anxiety-like behavior, learning, and sensitivity to stimulants. Additional experiments showed that these alterations were associated with an overall increased activity of striatal neurons. Thus, the small population of nicotinic receptors represents an interesting target for a modulation of response to stimulant drugs and other striatal-based behavior.
Keywords: Fos; acetylcholine; behavior; interneurons; nAChRs; striatum.
Copyright © 2022 the authors.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
