Metabolic syndrome is associated with poor response to rifaximin in minimal hepatic encephalopathy
- PMID: 35165326
- PMCID: PMC8844048
- DOI: 10.1038/s41598-022-06416-z
Metabolic syndrome is associated with poor response to rifaximin in minimal hepatic encephalopathy
Abstract
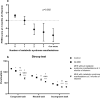
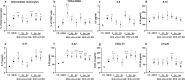
Patients with cirrhosis may show minimal hepatic encephalopathy (MHE), for which rifaximin is effective. Metabolic syndrome may be associated with cognitive impairment. Our aims were to evaluate the influence of metabolic syndrome features on response to rifaximin for neurological and inflammatory alterations in MHE. A prospective cohort study was conducted in 63 cirrhotic patients and 30 controls from two tertiary centres recruited between 2015 and 2019. Metabolic syndrome was defined according to the Adult Treatment Panel-III. Patients were classified into 31 without and 32 with MHE according to the Psychometric Hepatic Encephalopathy Score (PHES). All participants performed specific psychometric tests, and inflammatory parameters were studied. Patients with MHE received rifaximin (400 mg/8 h). Response was evaluated by PHES at 3 and 6 months. Response according to metabolic syndrome manifestations was compared. The response rate was 66%. Older age (p = 0.012) and all metabolic syndrome diseases (p < 0.05) were associated with non-response, plus an increase in risk as the number of manifestations rose (p < 0.001). Patients with metabolic manifestations exhibited worse processing speed (p = 0.011), working memory (p = 0.005), visual coordination (p = 0.013) and lower proportion of activated CD4+ lymphocytes (p = 0.039) at baseline, as well as worse concentration (p = 0.030), bimanual coordination (p = 0.004) and higher levels of intermediate monocytes (p = 0.026), CX3CL1 (p < 0.05), IL-17 (p = 0.022), AHR (p = 0.010) and IgG (p < 0.05) at 3 and/or 6 months of rifaximin. Patients with clinical signs of metabolic syndrome have poor response to rifaximin for MHE, with a higher proportion of neurological alterations associated with a pro-inflammatory environment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Goyal O, Sidhu S, Kishore H. Incidence, prevalence and natural history of minimal hepatic encephalopathy in cirrhosis. J. Hepatol. 2016;64:S279. doi: 10.1016/S0168-8278(16)00334-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

