Impaired phosphocreatine metabolism in white adipocytes promotes inflammation
- PMID: 35165448
- PMCID: PMC8885409
- DOI: 10.1038/s42255-022-00525-9
Impaired phosphocreatine metabolism in white adipocytes promotes inflammation
Abstract
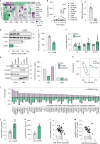
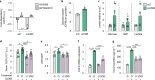
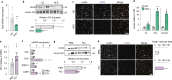
The mechanisms promoting disturbed white adipocyte function in obesity remain largely unclear. Herein, we integrate white adipose tissue (WAT) metabolomic and transcriptomic data from clinical cohorts and find that the WAT phosphocreatine/creatine ratio is increased and creatine kinase-B expression and activity is decreased in the obese state. In human in vitro and murine in vivo models, we demonstrate that decreased phosphocreatine metabolism in white adipocytes alters adenosine monophosphate-activated protein kinase activity via effects on adenosine triphosphate/adenosine diphosphate levels, independently of WAT beigeing. This disturbance promotes a pro-inflammatory profile characterized, in part, by increased chemokine (C-C motif) ligand 2 (CCL2) production. These data suggest that the phosphocreatine/creatine system links cellular energy shuttling with pro-inflammatory responses in human and murine white adipocytes. Our findings provide unexpected perspectives on the mechanisms driving WAT inflammation in obesity and may present avenues to target adipocyte dysfunction.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












Comment in
-
A role for phosphocreatine in adipose inflammation.Nat Rev Endocrinol. 2022 May;18(5):266. doi: 10.1038/s41574-022-00654-x. Nat Rev Endocrinol. 2022. PMID: 35217795 No abstract available.
References
-
- Mejhert N, Ryden M. Novel aspects on the role of white adipose tissue in type 2 diabetes. Curr. Opin. Pharmacol. 2020;55:47–52. - PubMed
-
- Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. - PubMed
-
- Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017;13:633–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

