Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines
- PMID: 35165453
- PMCID: PMC9117148
- DOI: 10.1038/s41591-022-01721-6
Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines
Abstract
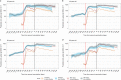
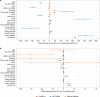
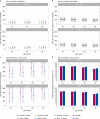
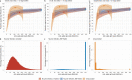
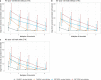
Antibody responses are an important part of immunity after Coronavirus Disease 2019 (COVID-19) vaccination. However, antibody trajectories and the associated duration of protection after a second vaccine dose remain unclear. In this study, we investigated anti-spike IgG antibody responses and correlates of protection after second doses of ChAdOx1 or BNT162b2 vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the United Kingdom general population. In 222,493 individuals, we found significant boosting of anti-spike IgG by the second doses of both vaccines in all ages and using different dosing intervals, including the 3-week interval for BNT162b2. After second vaccination, BNT162b2 generated higher peak levels than ChAdOX1. Older individuals and males had lower peak levels with BNT162b2 but not ChAdOx1, whereas declines were similar across ages and sexes with ChAdOX1 or BNT162b2. Prior infection significantly increased antibody peak level and half-life with both vaccines. Anti-spike IgG levels were associated with protection from infection after vaccination and, to an even greater degree, after prior infection. At least 67% protection against infection was estimated to last for 2-3 months after two ChAdOx1 doses, for 5-8 months after two BNT162b2 doses in those without prior infection and for 1-2 years for those unvaccinated after natural infection. A third booster dose might be needed, prioritized to ChAdOx1 recipients and those more clinically vulnerable.
© 2022. The Author(s).
Conflict of interest statement
D.W.E. declares lecture fees from Gilead, outside the submitted work. No other author has a conflict of interest to declare.
Figures










References
-
- Medicines and Healthcare products Regulatory Agency. Regulatory approval of Pfizer/BioNTech vaccine for COVID-19. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer... (2020).
-
- Medicines and Healthcare products Regulatory Agency. Oxford University/AstraZeneca COVID-19 vaccine approved. https://www.gov.uk/government/news/oxford-universityastrazeneca-covid-19... (2020).
-
- Department of Health and Social Care. UK COVID-19 vaccines delivery plan. https://www.gov.uk/government/publications/uk-covid-19-vaccines-delivery... (2021).
-
- Vaccinations in United Kingdom. https://coronavirus.data.gov.uk/details/vaccinations (2021).
-
- Department of Health and Social Care. Prioritising the first COVID-19 vaccine dose: JCVI statement. https://www.gov.uk/government/publications/prioritising-the-first-covid-... (2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

