Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study
- PMID: 35165669
- PMCID: PMC8828370
- DOI: 10.1016/S2666-5247(21)00305-0
Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study
Abstract
Background: Vaccination is an efficient strategy to control the COVID-19 pandemic. In north Cyprus, vaccine distribution started with CoronaVac followed by BNT162b2, and ChAdOx1 vaccines. An option to obtain a third booster dose with BNT162b2 or CoronaVac was later offered to people fully inoculated with CoronaVac. There are few simultaneous and comparative real-world antibody data for these three vaccines as well as boosters after CoronaVac vaccination. Our study was aimed at evaluating antibody responses after these vaccination schemes.
Methods: We did a prospective, longitudinal population-based study to measure SARS-CoV-2 anti-spike receptor binding domain (RBD) IgG concentrations, assessed by assaying blood samples collected, in participants in north Cyprus who had received the BNT162b2, ChAdOx1, or CoronaVac vaccine at 1 month and 3 months after the second dose. Participants were recruited when they voluntarily came to the laboratory for testing after vaccination, solicited from health-care access points, or from the general population. We also evaluated antibody responses 1 month after a booster dose of BNT162b2 or CoronaVac after primary CoronaVac regimen. Demographics, baseline characteristics, vaccination reactions, and percentage of antibody responders were collected by phone interviews or directly from the laboratory summarised by vaccine and age group. Antibody levels were compared between groups over time by parametric and non-parametric methods.
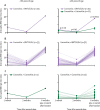
Findings: Recruitment, follow-up, and data collection was done between March 1 and Sept 30, 2021. BNT162b2 induced the highest seropositivity and anti-spike RBD IgG antibody titres, followed by ChAdOx1, and then by CoronaVac. In addition, the rate of decline of antibodies was fastest with CoronaVac, followed by ChAdOx1, and then by BNT162b2. For the older age group, the rate of seropositivity at 3 months after the second dose was 100% for BNT162b2, 90% for ChAdOx1, and 60% for CoronaVac. In the multivariate repeated measures model, lower antibody titres were also significantly associated with male sex, older age, and time since vaccination. Boosting a two-dose CoronaVac regimen at 6 months with a single BNT162b2 dose led to significantly increased titres of IgG compared with boosting with CoronaVac; for the 60 years and older age group, the geometric mean fold rise in antibody titre after the booster relative to 1 month post-baseline was 7·9 (95% CI 5·8-10·8) in the BNT162b2 boost group versus 2·8 (1·6-5·0) in the CoronaVac group.
Interpretation: These longitudinal data can help shape vaccination strategies. Given the low antibody titres and fast decline in the CoronaVac group in individuals 60 years or older, more potent vaccine options could be considered as the primary vaccination or booster dose in these high-risk populations to sustain antibody responses for longer.
Funding: Crowdfunded in north Cyprus.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
BB is currently an employee of The Emmes Company. The Emmes Company did not fund the study, nor did it play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. ÖU is currently an employee of the Novartis Institutes for Biomedical Research. This study was done entirely independently from Novartis. Novartis did not request, authorise, or fund the study, nor did it play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views and opinions expressed in this publication are those of the authors and do not necessarily reflect the official policy or position of Novartis or any of its officers. All other authors declare no competing interests.
Figures

Similar articles
-
Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia.Lancet Infect Dis. 2023 May;23(5):545-555. doi: 10.1016/S1473-3099(22)00800-3. Epub 2023 Jan 11. Lancet Infect Dis. 2023. PMID: 36640798 Clinical Trial.
-
Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study.Lancet. 2022 Feb 5;399(10324):521-529. doi: 10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. Lancet. 2022. PMID: 35074136 Free PMC article. Clinical Trial.
-
COVID-19 lateral flow IgG seropositivity and serum neutralising antibody responses after primary and booster vaccinations in Chile: a cross-sectional study.Lancet Microbe. 2023 Mar;4(3):e149-e158. doi: 10.1016/S2666-5247(22)00290-7. Epub 2023 Jan 27. Lancet Microbe. 2023. PMID: 36716754 Free PMC article.
-
Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD015021. doi: 10.1002/14651858.CD015021. Cochrane Database Syst Rev. 2022. PMID: 35943061 Free PMC article.
-
Longevity of immunity following COVID-19 vaccination: a comprehensive review of the currently approved vaccines.Hum Vaccin Immunother. 2022 Nov 30;18(5):2037384. doi: 10.1080/21645515.2022.2037384. Epub 2022 Apr 13. Hum Vaccin Immunother. 2022. PMID: 35417285 Free PMC article. Review.
Cited by
-
Population-Based Analysis of the Immunoglobulin G Response to Different COVID-19 Vaccines in Brazil.Vaccines (Basel). 2022 Dec 22;11(1):21. doi: 10.3390/vaccines11010021. Vaccines (Basel). 2022. PMID: 36679871 Free PMC article.
-
Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in People with Diabetes: A Prospective Observational Study.Vaccines (Basel). 2022 Mar 2;10(3):382. doi: 10.3390/vaccines10030382. Vaccines (Basel). 2022. PMID: 35335014 Free PMC article.
-
Impact of vaccine platform and BCG vaccination on antibody responses to COVID-19 vaccination.Front Immunol. 2023 Jul 3;14:1172851. doi: 10.3389/fimmu.2023.1172851. eCollection 2023. Front Immunol. 2023. PMID: 37465688 Free PMC article. Clinical Trial.
-
Effectiveness of the BNT162b2 and the CoronaVac vaccines and boosters in healthcare workers.Hum Vaccin Immunother. 2023 Dec 15;19(3):2275445. doi: 10.1080/21645515.2023.2275445. Epub 2023 Nov 15. Hum Vaccin Immunother. 2023. PMID: 37964650 Free PMC article.
-
The safety and immunogenicity to inactivated COVID-19 vaccine in patients with hyperlipemia.Open Med (Wars). 2023 Aug 30;18(1):20230780. doi: 10.1515/med-2023-0780. eCollection 2023. Open Med (Wars). 2023. PMID: 37693840 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

