Population-based screening in children for early diagnosis and treatment of familial hypercholesterolemia: design of the VRONI study
- PMID: 35165720
- PMCID: PMC9159326
- DOI: 10.1093/eurpub/ckac007
Population-based screening in children for early diagnosis and treatment of familial hypercholesterolemia: design of the VRONI study
Abstract
Background: Heterozygous familial hypercholesterolemia (FH) represents the most frequent monogenic disorder with an estimated prevalence of 1:250 in the general population. Diagnosis during childhood enables early initiation of preventive measures, reducing the risk of severe consecutive atherosclerotic manifestations. Nevertheless, population-based screening programs for FH are scarce.
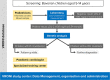
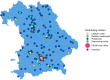
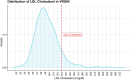
Methods: In the VRONI study, children aged 5-14 years in Bavaria are invited to participate in an FH screening program during regular pediatric visits. The screening is based on low-density lipoprotein cholesterol measurements from capillary blood. If exceeding 130 mg/dl (3.34 mmol/l), i.e. the expected 95th percentile in this age group, subsequent molecular genetic analysis for FH is performed. Children with FH pathogenic variants enter a registry and are treated by specialized pediatricians. Furthermore, qualified training centers offer FH-focused training courses to affected families. For first-degree relatives, reverse cascade screening is recommended to identify and treat affected family members.
Results: Implementation of VRONI required intensive prearrangements for addressing ethical, educational, data safety, legal and organizational aspects, which will be outlined in this article. Recruitment started in early 2021, within the first months, more than 380 pediatricians screened over 5200 children. Approximately 50 000 children are expected to be enrolled in the VRONI study until 2024.
Conclusions: VRONI aims to test the feasibility of a population-based screening for FH in children in Bavaria, intending to set the stage for a nationwide FH screening infrastructure. Furthermore, we aim to validate genetic variants of unclear significance, detect novel causative mutations and contribute to polygenic risk indices (DRKS00022140; August 2020).
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Public Health Association.
Figures

References
-
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol 2004;160:407–20. - PubMed
-
- Nordestgaard BG, Chapman MJ, Humphries SE, et al.; for the European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–90a. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

