Sec62 promotes gastric cancer metastasis through mediating UPR-induced autophagy activation
- PMID: 35165763
- PMCID: PMC11073224
- DOI: 10.1007/s00018-022-04143-2
Sec62 promotes gastric cancer metastasis through mediating UPR-induced autophagy activation
Abstract
Background and aims: Sec62 is a membrane protein of the endoplasmic reticulum that facilitates protein transport. Its role in cancer is increasingly recognised, but remains largely unknown. We investigated the functional role of Sec62 in gastric cancer (GC) and its underlying mechanism.
Methods: Bioinformatics, tissue microarray, immunohistochemistry (IHC), western blotting (WB), quantitative polymerase chain reaction (qPCR), and immunofluorescence were used to examine the expression of target genes. Transwell, scratch healing assays, and xenograft models were used to evaluate cell migration and invasion. Transmission electron microscopy and mRFP-GFP-LC3 double-labeled adenoviruses were used to monitor autophagy. Co-immunoprecipitation (CO-IP) was performed to evaluate the binding activity between the proteins.
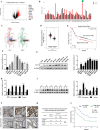
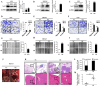
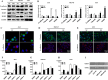
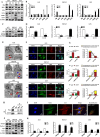
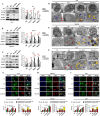
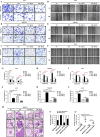
Results: Sec62 expression was upregulated in GC, and Sec62 upregulation was an independent predictor of poor prognosis. Sec62 overexpression promoted GC cell migration and invasion both in vitro and in vivo. Sec62 promoted migration and invasion by affecting TIMP-1 and MMP2/9 balance. Moreover, Sec62 could activate autophagy by upregulating PERK/ATF4 expression and binding to LC3II with concomitant FIP200/Beclin-1/Atg5 activation. Furthermore, autophagy blockage impaired the promotive effects of Sec62 on GC cell migration and invasion, whereas autophagy activation rescued the inhibitory effect of Sec62 knockdown on GC metastasis. Notably, Sec62 inhibition combined with autophagy blockage exerted a synergetic anti-metastatic effect in vitro and in vivo.
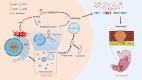
Conclusion: Sec62 promotes GC metastasis by activating autophagy and subsequently regulating TIMP-1 and MMP2/9 balance. The activation of autophagy by Sec62 may involve the unfolded protein response (UPR)-related PERK/ATF4 pathway and binding of LC3II during UPR recovery involving FIP200/Beclin-1/Atg5 upregulation. Specifically, the dual inhibition of Sec62 and autophagy may provide a promising therapeutic strategy for GC metastasis.
Keywords: EMT; ER stress; ER-phagy; Protein translocation machinery; Unfolded protein response (UPR).
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
None.
Figures
Similar articles
-
IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9.Mol Cancer. 2014 Jan 31;13:18. doi: 10.1186/1476-4598-13-18. Mol Cancer. 2014. PMID: 24479681 Free PMC article.
-
Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery.Nat Cell Biol. 2016 Nov;18(11):1173-1184. doi: 10.1038/ncb3423. Epub 2016 Oct 17. Nat Cell Biol. 2016. PMID: 27749824
-
The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress.J Biol Chem. 2019 May 17;294(20):8197-8217. doi: 10.1074/jbc.RA118.002829. Epub 2019 Mar 29. J Biol Chem. 2019. PMID: 30926605 Free PMC article.
-
Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles.Autophagy. 2021 Feb;17(2):385-401. doi: 10.1080/15548627.2020.1725377. Epub 2020 Feb 12. Autophagy. 2021. PMID: 32048886 Free PMC article. Review.
-
The endoplasmic reticulum membrane protein Sec62 as potential therapeutic target in SEC62 overexpressing tumors.Front Physiol. 2022 Oct 3;13:1014271. doi: 10.3389/fphys.2022.1014271. eCollection 2022. Front Physiol. 2022. PMID: 36262254 Free PMC article. Review.
Cited by
-
Endoplasmic reticulum-resident protein Sec62 drives colorectal cancer metastasis via MAPK/ATF2/UCA1 axis.Cell Prolif. 2022 Dec;55(12):e13253. doi: 10.1111/cpr.13253. Epub 2022 Oct 5. Cell Prolif. 2022. PMID: 36200182 Free PMC article.
-
Autophagy of the ER: the secretome finds the lysosome.FEBS J. 2023 Dec;290(24):5656-5673. doi: 10.1111/febs.16986. Epub 2023 Nov 3. FEBS J. 2023. PMID: 37920925 Free PMC article. Review.
-
ROS-mediated lysosomal membrane permeabilization and autophagy inhibition regulate bleomycin-induced cellular senescence.Autophagy. 2024 Sep;20(9):2000-2016. doi: 10.1080/15548627.2024.2353548. Epub 2024 May 18. Autophagy. 2024. PMID: 38762757 Free PMC article.
-
Long non‑coding RNA SNHG1 promotes autophagy in vascular smooth muscle cells induced by facilitating CLEC7A.Mol Med Rep. 2025 Jan;31(1):20. doi: 10.3892/mmr.2024.13385. Epub 2024 Nov 8. Mol Med Rep. 2025. PMID: 39513586 Free PMC article.
-
The dual effect of endoplasmic reticulum stress in digestive system tumors and intervention of Chinese botanical drug extracts: a review.Front Pharmacol. 2024 Feb 21;15:1339146. doi: 10.3389/fphar.2024.1339146. eCollection 2024. Front Pharmacol. 2024. PMID: 38449811 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray F(2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin - PubMed
-
- Fumagalli F, Noack J, Bergmann TJ, Cebollero E, Pisoni GB, Fasana E, Fregno I, Galli C, Loi M, Solda T, D'Antuono R, Raimondi A, Jung M, Melnyk A, Schorr S, Schreiber A, Simonelli L, Varani L, Wilson-Zbinden C, Zerbe O, Hofmann K, Peter M, Quadroni M, Zimmermann R, Molinari M. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173–1184. doi: 10.1038/ncb3423. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 81772650/national natural science foundation of china
- 81322037/national natural science foundation of china
- 81572302/national natural science foundation of china
- 81421003/national natural science foundation of china
- 201182/foundation for the author of national excellent doctoral dissertation of the people's republic of china
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

