Romosozumab and antiresorptive treatment: the importance of treatment sequence
- PMID: 35165774
- PMCID: PMC9106644
- DOI: 10.1007/s00198-021-06174-0
Romosozumab and antiresorptive treatment: the importance of treatment sequence
Abstract
To evaluate whether treatment sequence affects romosozumab response, this analysis reviewed studies where romosozumab was administered before or following an antiresorptive (alendronate or denosumab). Initial treatment with romosozumab followed by an antiresorptive resulted in larger increases in bone mineral density of both hip and spine compared with the reverse sequence.
Introduction: Teriparatide followed by an antiresorptive increases bone mineral density (BMD) more than using an antiresorptive first. To evaluate whether treatment sequence affects romosozumab response, we reviewed randomized clinical trials where romosozumab was administered before (ARCH, FRAME) or following (STRUCTURE, Phase 2 extension) an antiresorptive (alendronate or denosumab, respectively).
Methods: We evaluated BMD percentage change for total hip (TH) and lumbar spine (LS) and response rates (BMD gains ≥ 3% and ≥ 6%) at years 1 and 2 (except STRUCTURE with only 1-year data available).
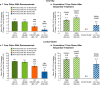
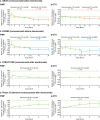
Results: With 1-year romosozumab initial therapy in ARCH and FRAME, TH BMD increased 6.2% and 6.0%, and LS BMD increased 13.7% and 13.1%, respectively. When romosozumab was administered for 1 year after alendronate (STRUCTURE) or denosumab (Phase 2 extension), TH BMD increased 2.9% and 0.9%, respectively, and LS BMD increased 9.8% and 5.3%, respectively. Over 2 years, TH and LS BMD increased 7.1% and 15.2% with romosozumab/alendronate, 8.5% and 16.6% with romosozumab/denosumab, and 3.8% and 11.5% with denosumab/romosozumab, respectively. A greater proportion of patients achieved BMD gains ≥ 6% when romosozumab was used first, particularly for TH, versus the reverse sequence (69% after romosozumab/denosumab; 15% after denosumab/romosozumab).
Conclusion: In this study, larger mean BMD increases and greater BMD responder rates were achieved when romosozumab was used before, versus after, an antiresorptive agent. Since BMD on treatment is a strong surrogate for bone strength and fracture risk, this analysis supports the thesis that initial treatment with romosozumab followed by an antiresorptive will result in greater efficacy versus the reverse sequence.
Keywords: Anabolic; Antiresorptive; Romosozumab; Teriparatide; Treatment sequence.
© 2022. The Author(s).
Conflict of interest statement
FC has received institutional grants and research support from Amgen, has served as a consultant for Amgen and Radius Health, and has served on the speakers’ bureaus for Amgen and Radius Health. DLK has received institutional grants and research support from Amgen and Radius Health; has served as a consultant for Amgen, Eli Lilly, and Pfizer; and has served on the speakers’ bureaus for Amgen and Eli Lilly. BLL has received institutional grants and research support from Amgen and Novo Nordisk; has served as a consultant for Amgen, UCB Pharma, Eli Lilly, Gedeon Richter, and Gilead; and has served on the speakers’ bureaus for Eli Lilly, Amgen, and UCB Pharma. BZL has received institutional grants and research support from Amgen and has served as a consultant for Amgen and Radius Health. EML has received research grants from Radius Health, Amgen, Mereo, and Bindex; income for service on scientific advisory boards or consulting for Amgen, Radius, Alexion, Sandoz, Samsung Bioepis, and Sanifit; income for service on speakers’ bureaus for Radius and Alexion; project development support for University of New Mexico; and royalties from UpToDate for sections on DXA, fracture risk assessment, and prevention of osteoporosis. He is a board member of the National Osteoporosis Foundation, International Society for Clinical Densitometry, and Osteoporosis Foundation of New Mexico. AM has served as a consultant for Amgen, Amgen Astellas BioPharma K.K., and Teijin Pharma. MR, MM, MKO, and CEM are employees and stockholders of Amgen. CL is an employee and stockholder of UCB Pharma. SF has received institutional grants and research support from Amgen, UCB Pharma, AgNovos, Alexion, and Labatec and has served as a consultant for Amgen, UCB Pharma, Alexion, AgNovos, Radius Health, and Labatec.
Figures

References
-
- Saag KG, Zanchetta JR, Devogelaer J-P, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six–month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–3355. doi: 10.1002/art.24879. - DOI - PubMed
-
- Hadji P, Zanchetta JR, Russo L, Recknor CP, Saag KG, McKiernan FE, Silverman SL, Alam J, Burge RT, Krege JH, Lakshmanan MC, Masica DN, Mitlak BH, Stock JL. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012;23:2141–2150. doi: 10.1007/s00198-011-1856-y. - DOI - PubMed
-
- Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Möricke R, López-Romero P. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–240. doi: 10.1016/S0140-6736(17)32137-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

