Antibody Therapies in Autoimmune Neuromuscular Junction Disorders: Approach to Myasthenic Crisis and Chronic Management
- PMID: 35165857
- PMCID: PMC9294078
- DOI: 10.1007/s13311-022-01181-3
Antibody Therapies in Autoimmune Neuromuscular Junction Disorders: Approach to Myasthenic Crisis and Chronic Management
Abstract
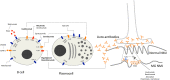
Myasthenia gravis (MG) is a neurological autoimmune disorder characterized by muscle weakness and fatigue. It is a B cell-mediated disease caused by pathogenic antibodies directed against various components of the neuromuscular junction (NMJ). Despite the wide range of adverse effects, current treatment is still based on non-specific immunosuppression, particularly on long-term steroid usage. The increasing knowledge regarding the pathogenic mechanisms of MG has however allowed to create more target-specific therapies. A very attractive therapeutic approach is currently offered by monoclonal antibodies (mAbs), given their ability to specifically and effectively target different immunopathological pathways, such as the complement cascade, B cell-related cluster of differentiation (CD) proteins, and the human neonatal Fc receptor (FcRn). Up to now, eculizumab, a C5-directed mAb, has been approved for the treatment of generalized MG (gMG) and efgartigimod, a FcRn inhibitor, has just been approved by the U.S. Food and Drug Administration for the treatment of anti-acetylcholine receptor (AChR) antibody positive gMG. Other mAbs are currently under investigation with encouraging preliminary results, further enriching the new range of therapeutic possibilities for MG. This review article provides an overview of the present status of mAb-based therapies for MG, which offer an exciting promise for better outcomes by setting the basis of a precision medicine approach.
Keywords: B cell; Biological drugs; Immunosuppression; Monoclonal antibody; Myasthenia gravis.
© 2022. The American Society for Experimental NeuroTherapeutics, Inc.
Figures
Comment in
-
What are the pharmacotherapeutic considerations for the treatment of myasthenia gravis?Expert Opin Pharmacother. 2022 Sep;23(13):1471-1474. doi: 10.1080/14656566.2022.2122710. Epub 2022 Sep 14. Expert Opin Pharmacother. 2022. PMID: 36104973 No abstract available.
References
-
- Vincent A, Huda S, Cao M, Cetin H, Koneczny I, Rodriguez-Cruz P, Jacobson L, Viegas S, Jacob S, Woodhall M, Nagaishi A. Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci. 2018;1413(1):143–153. - PubMed
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. The Lancet Neurology. 2015;14(10):1023–1036. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

