Synthesis of research on ENFit gastrostomy tubes with potential implications for US patients using these devices
- PMID: 35165940
- PMCID: PMC11472396
- DOI: 10.1002/ncp.10829
Synthesis of research on ENFit gastrostomy tubes with potential implications for US patients using these devices
Abstract
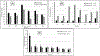
Misconnections between enteral devices and other medical devices have been associated with patient death and serious injuries. To minimize such misconnections, the design of connectors on enteral devices has been standardized. The most common adaptation of the standardized enteral connector is called ENFit. Gastrostomy tubes (G-tubes), which may or may not possess the ENFit connector, are increasingly used to deliver commercial and blenderized diets in home settings to enteral device users. To investigate and compare the performance of G-tubes with and without ENFit connectors, research investigations have recently been performed. However, synthesis of such investigations and quantitative discussion of the consequences of transitioning to ENFit-based G-tube devices has not yet occurred. Here we review the research findings from these studies, with data on patient practices from a Mayo Clinic survey, to estimate the impact on tube feeders in home settings of transitioning to ENFit-based G-tube devices. Extrapolating the findings from these studies to US enteral G-tube patients, 1.5%-8.6% of adult patients and 0.2%-1.9% of pediatric patients may experience perceptible slowing in their gravity feeds if using ENFit-based G-tube devices. About 2.5%-8.6% of adult patients and 0.5%-5.5% of pediatric patients (or their caregivers) may need to push with perceptibly more force for syringe push-based feeding using ENFit-based G-tube devices. Lastly, the article offers suggestions for patients and device manufacturers. [Correction added on 2 May 2022, after first online publication: In the preceding sentence, the percentage of adult patients was revised from 2.5%-8.6% to 1.5%-8.6%.].
Keywords: ENFit; blenderized diets; enteral nutrition; gastrostromy tube; misconnections.
© 2022 American Society for Parenteral and Enteral Nutrition.
Conflict of interest statement
CONFLICT OF INTEREST
None declared.
Figures




Similar articles
-
Impact of Design Changes in Gastrostomy Tube (G-tube) Devices for Patients Who Rely on Home-Based Blenderized Diets for Enteral Nutrition.J Am Coll Nutr. 2019 May-Jun;38(4):311-317. doi: 10.1080/07315724.2018.1509247. Epub 2019 Mar 1. J Am Coll Nutr. 2019. PMID: 30821589
-
In Vitro Performance Testing of Legacy and ENFit Gastrostomy Tube Devices Under Gravity Flow Conditions.JPEN J Parenter Enteral Nutr. 2018 Nov;42(8):1334-1341. doi: 10.1002/jpen.1159. Epub 2018 Apr 27. JPEN J Parenter Enteral Nutr. 2018. PMID: 29701924
-
Gravity Flow in Proposed Enteral Tube Small-Bore Connectors.Nutr Clin Pract. 2017 Apr;32(2):189-192. doi: 10.1177/0884533616679141. Epub 2016 Dec 5. Nutr Clin Pract. 2017. PMID: 27913774
-
The lingering safety menace: A 10-year review of enteral misconnection adverse events and narrative review.Nutr Clin Pract. 2024 Oct;39(5):1251-1258. doi: 10.1002/ncp.11191. Epub 2024 Jul 18. Nutr Clin Pract. 2024. PMID: 39023510 Review.
-
ENFit Enteral Nutrition Connectors.Nutr Clin Pract. 2016 Dec;31(6):769-772. doi: 10.1177/0884533616673638. Epub 2016 Oct 22. Nutr Clin Pract. 2016. PMID: 27756849 Review.
Cited by
-
Oral Drug Product Administration via Enteral Feeding Tubes: In Vitro Testing.AAPS J. 2024 Apr 4;26(3):43. doi: 10.1208/s12248-024-00896-9. AAPS J. 2024. PMID: 38575754
References
-
- Guenter P, Hicks RW, Simmons D, et al. Enteral feeding misconnections: a consortium position statement. Jt Comm J Qual Patient Saf. 2008;34(5):285–292. - PubMed
-
- U. S. Food and Drug Administration. Accessed January 10, 2020. https://www.fda.gov/medical-devices/medical-device-connectors/reducing-r...
-
- Guenter P, Lyman B. ENFit enteral nutrition connectors: benefits and challenges. Nutr Clin Pract. 2016;31(6):769–772. - PubMed
-
- Simmons D, Symes L, Graves K. Tubing misconnections: normalization of deviance. Nutr Clin Pract. 2011;26(3):286–293. - PubMed
-
- Hurt RT, Epp LM, Pattison AK, et al. Gravity flow in proposed enteral tube small-bore connectors. Nutr Clin Pract. 2016;32(2):189–192. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

