Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study
- PMID: 35166043
- PMCID: PMC8977152
- DOI: 10.1111/1759-7714.14351
Microwave ablation via a flexible catheter for the treatment of nonsurgical peripheral lung cancer: A pilot study
Abstract
Background: Endobronchial microwave ablation via flexible catheter offers the potential for local therapy for inoperable peripheral lung cancer. The study aimed to evaluate the feasibility and safety of navigation bronchoscopy-guided water-cooled microwave ablation catheter for nonsurgical peripheral lung cancer.
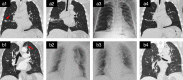
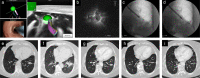
Methods: This was a prospective single arm pilot study. Patients with early stage or multiple primary peripheral lung cancer who were nonsurgical candidates for surgery were enrolled in the study. Bronchoscopic microwave ablation was performed via a flexible water-cooled microwave ablation antenna under the guidance of navigation bronchoscopy. Radial probe endobronchial ultrasound combined with fluoroscopy was used to confirm the position. Treatment outcomes were evaluated based on follow-up chest CT and positron emission tomography scans. Primary endpoints were technical success and safety. Secondary endpoints were complete ablation rate, 2-year local control rate, and progression-free survival.
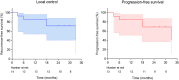
Results: Thirteen patients were enrolled in the study from April 2018 to July 2019. A total of 19 sessions of microwave ablation were performed on 14 tumors under the guidance of navigation bronchoscopy. The technical success was 100%. Treatment-related complications occurred in two patients. The complete ablation rate was 78.6% (11/14). The 2-year local control rate was 71.4%. Median progression-free survival was 33 months for all patients.
Conclusions: In this pilot study, bronchoscopic microwave ablation appears to be feasible with acceptable occurrence of complication in the treatment of peripheral lung cancer under the guidance of navigation bronchoscopy.
Trial registration: ClinicalTrials.gov NCT02972177.
Keywords: bronchoscopic therapy; lung cancer; microwave ablation; multiple primary lung cancer; navigation bronchoscopy.
© 2022 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. Shanghai Chest Hospital and Canyon Medical Inc. jointly own a patent of transbronchial microwave ablation antenna (patent No. ZL 201610424613.7). Dr Sun is the first inventor of this patent. The patent has at present been exclusively licensed to Canyon. The current study evaluates the antenna produced under license of this patent as well as commercialized product from competitor. The efficacy was measured as a combined evaluation of microwave ablation antenna and was not intended to compare the products from competitors. None of the authors holds equity of Canyon Medical Inc., nor are they employed by Canyon Medical Inc.
Figures
Similar articles
-
Novel Image-Guided Flexible-Probe Transbronchial Microwave Ablation for Stage 1 Lung Cancer.Respiration. 2023;102(3):182-193. doi: 10.1159/000528820. Epub 2023 Jan 18. Respiration. 2023. PMID: 36652940 Free PMC article.
-
The safety and feasibility of three-dimensional visualization planning system for CT-guided microwave ablation of stage I NSCLC (diameter ≤2.5 cm): A pilot study.J Cancer Res Ther. 2023 Feb;19(1):64-70. doi: 10.4103/jcrt.jcrt_2093_22. J Cancer Res Ther. 2023. PMID: 37006044
-
High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study.J Med Imaging Radiat Oncol. 2013 Aug;57(4):466-74. doi: 10.1111/1754-9485.12068. Epub 2013 May 8. J Med Imaging Radiat Oncol. 2013. PMID: 23870347 Clinical Trial.
-
Microwave Ablation for Malignant Central Airway Obstruction: A Pilot Study.Respiration. 2022;101(7):666-674. doi: 10.1159/000522544. Epub 2022 Mar 22. Respiration. 2022. PMID: 35316812 Free PMC article. Review.
-
Microwave ablation in the treatment of primary lung tumors.Semin Respir Crit Care Med. 2008 Aug;29(4):384-94. doi: 10.1055/s-2008-1081281. Semin Respir Crit Care Med. 2008. PMID: 18651356 Review.
Cited by
-
ERS International Congress 2020: highlights from the Clinical Techniques, Imaging and Endoscopy assembly.ERJ Open Res. 2021 May 31;7(2):00118-2021. doi: 10.1183/23120541.00118-2021. eCollection 2021 Apr. ERJ Open Res. 2021. PMID: 34084779 Free PMC article. Review.
-
Transbronchial cryoablation in peripheral lung parenchyma with a novel thin cryoprobe and initial clinical testing.Thorax. 2024 Jun 14;79(7):633-643. doi: 10.1136/thorax-2023-220227. Thorax. 2024. PMID: 38242710 Free PMC article. Clinical Trial.
-
Review on endobronchial therapies-current status and future.Ann Transl Med. 2024 Aug 1;12(4):75. doi: 10.21037/atm-23-1430. Epub 2023 Aug 11. Ann Transl Med. 2024. PMID: 39118957 Free PMC article. Review.
-
[Advances in the Treatment of Multiple Primary Lung Cancer].Zhongguo Fei Ai Za Zhi. 2025 Jun 20;28(6):460-466. doi: 10.3779/j.issn.1009-3419.2025.102.17. Zhongguo Fei Ai Za Zhi. 2025. PMID: 40640096 Free PMC article. Review. Chinese.
-
Advanced Imaging for Robotic Bronchoscopy: A Review.Diagnostics (Basel). 2023 Mar 5;13(5):990. doi: 10.3390/diagnostics13050990. Diagnostics (Basel). 2023. PMID: 36900134 Free PMC article. Review.
References
-
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non‐small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143:e278S–313S. - PubMed
-
- Erhunmwunsee L, D'Amico TA. Surgical management of pulmonary metastases. Ann Thorac Surg. 2009;88:2052–60. - PubMed
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology. Non‐Small Cell Lung Cancer. Available from https://www.nccn.org/professionals/physician_gls/default.aspx#nscl; Accessed February 29, 2020.
-
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early‐stage lung cancer. J Clin Oncol. 2006;24:4833–9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

