Age-related GSK3β overexpression drives podocyte senescence and glomerular aging
- PMID: 35166234
- PMCID: PMC8843754
- DOI: 10.1172/JCI141848
Age-related GSK3β overexpression drives podocyte senescence and glomerular aging
Abstract
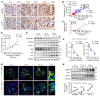
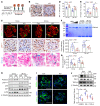
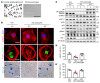
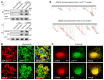
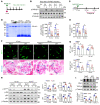
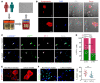
As life expectancy continues to increase, clinicians are challenged by age-related renal impairment that involves podocyte senescence and glomerulosclerosis. There is now compelling evidence that lithium has a potent antiaging activity that ameliorates brain aging and increases longevity in Drosophila and Caenorhabditis elegans. As the major molecular target of lithium action and a multitasking protein kinase recently implicated in a variety of renal diseases, glycogen synthase kinase 3β (GSK3β) is overexpressed and hyperactive with age in glomerular podocytes, correlating with functional and histological signs of kidney aging. Moreover, podocyte-specific ablation of GSK3β substantially attenuated podocyte senescence and glomerular aging in mice. Mechanistically, key mediators of senescence signaling, such as p16INK4A and p53, contain high numbers of GSK3β consensus motifs, physically interact with GSK3β, and act as its putative substrates. In addition, therapeutic targeting of GSK3β by microdose lithium later in life reduced senescence signaling and delayed kidney aging in mice. Furthermore, in psychiatric patients, lithium carbonate therapy inhibited GSK3β activity and mitigated senescence signaling in urinary exfoliated podocytes and was associated with preservation of kidney function. Thus, GSK3β appears to play a key role in podocyte senescence by modulating senescence signaling and may be an actionable senostatic target to delay kidney aging.
Keywords: Aging; Bipolar disorder; Cellular senescence; Cytoskeleton; Nephrology.
Figures


Comment in
-
GSK3β and the aging kidney.J Clin Invest. 2022 Feb 15;132(4):e155885. doi: 10.1172/JCI155885. J Clin Invest. 2022. PMID: 35166232 Free PMC article.
-
GSKβ as a target in podocyte aging.Kidney Int. 2022 Sep;102(3):463-465. doi: 10.1016/j.kint.2022.04.041. Epub 2022 Jun 1. Kidney Int. 2022. PMID: 35660495 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

