LPAR1 regulates enteric nervous system function through glial signaling and contributes to chronic intestinal pseudo-obstruction
- PMID: 35166239
- PMCID: PMC8843750
- DOI: 10.1172/JCI149464
LPAR1 regulates enteric nervous system function through glial signaling and contributes to chronic intestinal pseudo-obstruction
Abstract
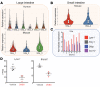
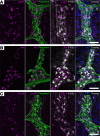
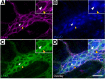
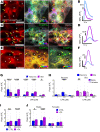
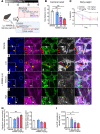
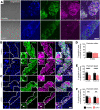
Gastrointestinal motility disorders involve alterations to the structure and/or function of the enteric nervous system (ENS) but the causal mechanisms remain unresolved in most cases. Homeostasis and disease in the ENS are processes that are regulated by enteric glia. Signaling mediated through type I lysophosphatidic acid receptors (LPAR1) has recently emerged as an important mechanism that contributes to disease, in part, through effects on peripheral glial survival and function. Enteric glia express LPAR1 but its role in ENS function and motility disorders is unknown. We used a combination of genetic, immunohistochemical, calcium imaging, and in vivo pharmacological approaches to investigate the role of LPAR1 in enteric glia. LPAR1 was enriched in enteric glia in mice and humans and LPA stimulated intracellular calcium responses in enteric glia, subsequently recruiting activity in a subpopulation of myenteric neurons. Blocking LPAR1 in vivo with AM966 attenuated gastrointestinal motility in mice and produced marked enteric neuro- and gliopathy. Samples from humans with chronic intestinal pseudo-obstruction (CIPO), a severe motility disorder, showed reduced glial LPAR1 expression in the colon and ileum. These data suggest that enteric glial LPAR1 signaling regulates gastrointestinal motility through enteric glia and could contribute to severe motility disorders in humans such as CIPO.
Keywords: Calcium signaling; G protein–coupled receptors; Gastroenterology; Homeostasis; Neuroscience.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

