A Nationwide Prospective Clinical Trial on Active Surveillance in Patients With Non-intraabdominal Desmoid-type Fibromatosis: The GRAFITI Trial
- PMID: 35166264
- PMCID: PMC9994811
- DOI: 10.1097/SLA.0000000000005415
A Nationwide Prospective Clinical Trial on Active Surveillance in Patients With Non-intraabdominal Desmoid-type Fibromatosis: The GRAFITI Trial
Erratum in
-
A Nationwide Prospective Clinical Trial on Active Surveillance in Patients With Non-intraabdominal Desmoid-type Fibromatosis: The GRAFITI Trial: Erratum.Ann Surg. 2023 Oct 1;278(4):e911. doi: 10.1097/SLA.0000000000006072. Epub 2023 Sep 7. Ann Surg. 2023. PMID: 37678388 Free PMC article. No abstract available.
Abstract
Objective: To assess tumor behavior and the efficacy of active surveillance (AS) in patients with desmoid-type fibromatosis (DTF).
Summary of background data: AS is recommended as initial management for DTF patients. Prospective data regarding the results of AS are lacking.
Methods: In this multicenter prospective cohort study (NTR4714), adult patients with non-intraabdominal DTF were followed during an initial AS approach for 3 years. Tumor behavior was evaluated according to Response Evaluation Criteria in Solid Tumors. Cumulative incidence of the start of an active treatment and progression-free survival (PFS) were calculated using the Kaplan-Meier method. Factors predictive for start of active treatment were assessed by Cox regression analyses.
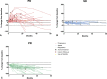
Results: A total of 105 patients started with AS. Median tumor size at baseline was 4.1cm (interquartile range 3.0-6.6). Fifty-seven patients had a T41A CTNNB1 mutation; 14 patients a S45F CTNNB1 mutation. At 3 years, cumulative incidence of the start of active treatment was 30% (95% confidence interval [CI] 21-39) and PFS was 58% (95% CI 49-69). Median time to start active treatment and PFS were not reached at a median follow-up of 33.7 months. During AS, 32% of patients had stable disease, 28% regressed, and 40% demonstrated initial progression. Larger tumor size (≥5 cm; hazard ratio = 2.38 [95% CI 1.15-4.90]) and S45F mutation (hazard ratio = 6.24 [95% CI 1.92-20.30]) were associated with the start of active treatment.
Conclusions: The majority DTF patients undergoing AS do not need an active treatment and experience stable or regressive disease, even after initial progression. Knowledge about the natural behavior of DTF will help to tailor the follow-up schedule to the individual patient.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors report no conflicts of interest and funding.
Figures

References
-
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, WHO Classification of Tumours. Geneva, Switzerland: WHO Press; 2020
-
- Kasper B, Raut CP, Gronchi A. Desmoid tumors: to treat or not to treat, that is the question. Cancer. 2020;126:5213–5221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

