Maternal plasma lipids are involved in the pathogenesis of preterm birth
- PMID: 35166340
- PMCID: PMC8847704
- DOI: 10.1093/gigascience/giac004
Maternal plasma lipids are involved in the pathogenesis of preterm birth
Abstract
Background: Preterm birth is defined by the onset of labor at a gestational age shorter than 37 weeks, and it can lead to premature birth and impose a threat to newborns' health. The Puerto Rico PROTECT cohort is a well-characterized prospective birth cohort that was designed to investigate environmental and social contributors to preterm birth in Puerto Rico, where preterm birth rates have been elevated in recent decades. To elucidate possible relationships between metabolites and preterm birth in this cohort, we conducted a nested case-control study to conduct untargeted metabolomic characterization of maternal plasma of 31 women who experienced preterm birth and 69 controls who underwent full-term labor at 24-28 gestational weeks.
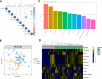
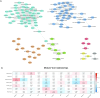
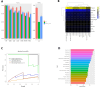
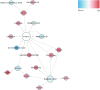
Results: A total of 333 metabolites were identified and annotated with liquid chromatography/mass spectrometry. Subsequent weighted gene correlation network analysis shows that the fatty acid and carene-enriched module has a significant positive association (P = 8e-04, FDR = 0.006) with preterm birth. After controlling for potential clinical confounders, a total of 38 metabolites demonstrated significant changes uniquely associated with preterm birth, where 17 of them were preterm biomarkers. Among 7 machine-learning classifiers, the application of random forest achieved a highly accurate and specific prediction (AUC = 0.92) for preterm birth in testing data, demonstrating their strong potential as biomarkers for preterm births. The 17 preterm biomarkers are involved in cell signaling, lipid metabolism, and lipid peroxidation functions. Additional modeling using only the 19 spontaneous preterm births (sPTB) and controls identifies 16 sPTB markers, with an AUC of 0.89 in testing data. Half of the sPTB overlap with those markers for preterm births. Further causality analysis infers that suberic acid upregulates several fatty acids to promote preterm birth.
Conclusions: Altogether, this study demonstrates the involvement of lipids, particularly fatty acids, in the pathogenesis of preterm birth.
Keywords: biomarkers; fatty acid; lipid; metabolic pathway; metabolomics; network; preterm.
© The Author(s) 2022. Published by Oxford University Press GigaScience.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Prediction of spontaneous preterm birth using supervised machine learning on metabolomic data: A case-cohort study.BJOG. 2024 Jun;131(7):908-916. doi: 10.1111/1471-0528.17723. Epub 2023 Nov 20. BJOG. 2024. PMID: 37984426
-
Metabolomic profiles of mid-trimester amniotic fluid are not associated with subsequent spontaneous preterm delivery or gestational duration at delivery.J Matern Fetal Neonatal Med. 2022 Jun;35(11):2054-2062. doi: 10.1080/14767058.2020.1777271. Epub 2020 Jun 16. J Matern Fetal Neonatal Med. 2022. PMID: 32543931
-
The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth.Int J Mol Sci. 2020 Feb 4;21(3):1043. doi: 10.3390/ijms21031043. Int J Mol Sci. 2020. PMID: 32033212 Free PMC article.
-
Omega-3 fatty acid addition during pregnancy.Cochrane Database Syst Rev. 2018 Nov 15;11(11):CD003402. doi: 10.1002/14651858.CD003402.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480773 Free PMC article.
-
Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies.Ultrasound Obstet Gynecol. 2018 Jan;51(1):43-53. doi: 10.1002/uog.18930. Ultrasound Obstet Gynecol. 2018. PMID: 29114987 Review.
Cited by
-
Fatty Acid Metabolism Disruptions: A Subtle yet Critical Factor in Adverse Pregnancy Outcomes.Int J Biol Sci. 2024 Nov 4;20(15):6018-6037. doi: 10.7150/ijbs.103404. eCollection 2024. Int J Biol Sci. 2024. PMID: 39664564 Free PMC article. Review.
-
First Trimester Prediction of Preterm Birth in Patient Plasma with Machine-Learning-Guided Raman Spectroscopy and Metabolomics.ACS Appl Mater Interfaces. 2023 Aug 16;15(32):38185-38200. doi: 10.1021/acsami.3c04260. Epub 2023 Aug 7. ACS Appl Mater Interfaces. 2023. PMID: 37549133 Free PMC article.
-
Machine learning applied in maternal and fetal health: a narrative review focused on pregnancy diseases and complications.Front Endocrinol (Lausanne). 2023 May 19;14:1130139. doi: 10.3389/fendo.2023.1130139. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37274341 Free PMC article. Review.
-
Associations of a Prenatal Serum Per- and Polyfluoroalkyl Substance Mixture with the Cord Serum Metabolome in the HOME Study.Environ Sci Technol. 2023 Dec 26;57(51):21627-21636. doi: 10.1021/acs.est.3c07515. Epub 2023 Dec 13. Environ Sci Technol. 2023. PMID: 38091497 Free PMC article.
-
Pre-pregnancy obesity is associated with an altered maternal metabolome and reduced Flt3L expression in preterm birth.Sci Rep. 2024 Dec 3;14(1):30027. doi: 10.1038/s41598-024-81194-4. Sci Rep. 2024. PMID: 39627409 Free PMC article.
References
-
- Callaghan WM, MacDorman MF, Rasmussen SA, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–73. - PubMed
-
- Cleary-Goldman J, Malone FD, Vidaver J, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105(5, Part 1):983–90. - PubMed
-
- Thayamballi N, Habiba S, Laribi O, et al. Impact of maternal demographic and socioeconomic factors on the association between particulate matter and adverse birth outcomes: a systematic review and meta-analysis. J Racial Ethn Health Disparities. 2021;8(3):743–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

