Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn's Disease Patients: The SUSTAIN Study
- PMID: 35166347
- PMCID: PMC9629456
- DOI: 10.1093/ibd/izab357
Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn's Disease Patients: The SUSTAIN Study
Abstract
Background: Large real-world-evidence studies are required to confirm the durability of response, effectiveness, and safety of ustekinumab in Crohn's disease (CD) patients in real-world clinical practice.
Methods: A retrospective, multicentre study was conducted in Spain in patients with active CD who had received ≥1 intravenous dose of ustekinumab for ≥6 months. Primary outcome was ustekinumab retention rate; secondary outcomes were to identify predictive factors for drug retention, short-term remission (week 16), loss of response and predictive factors for short-term efficacy and loss of response, and ustekinumab safety.
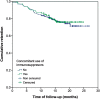
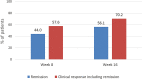
Results: A total of 463 patients were included. Mean baseline Harvey-Bradshaw Index was 8.4. A total of 447 (96.5%) patients had received prior biologic therapy, 141 (30.5%) of whom had received ≥3 agents. In addition, 35.2% received concomitant immunosuppressants, and 47.1% had ≥1 abdominal surgery. At week 16, 56% had remission, 70% had response, and 26.1% required dose escalation or intensification; of these, 24.8% did not subsequently reduce dose. After a median follow-up of 15 months, 356 (77%) patients continued treatment. The incidence rate of ustekinumab discontinuation was 18% per patient-year of follow-up. Previous intestinal surgery and concomitant steroid treatment were associated with higher risk of ustekinumab discontinuation, while a maintenance schedule every 12 weeks had a lower risk; neither concomitant immunosuppressants nor the number of previous biologics were associated with ustekinumab discontinuation risk. Fifty adverse events were reported in 39 (8.4%) patients; 4 of them were severe (2 infections, 1 malignancy, and 1 fever).
Conclusions: Ustekinumab is effective and safe as short- and long-term treatment in a refractory cohort of CD patients in real-world clinical practice.
Keywords: Crohn’s disease; effectiveness; real-world evidence; safety; ustekinumab.
Plain language summary
This large retrospective study demonstrated the short- and long-term effectiveness and safety of ustekinumab in patients with Crohn’s disease in real-world clinical practice, including those with refractory disease.
© 2022 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures
References
-
- Baumgart DC, Sandborn WJ.. Crohn’s disease. Lancet. 2012;380:1590–1605. - PubMed
-
- Veauthier B, Hornecker JR.. Crohn’s disease: diagnosis and management. Am Fam Physician. 2018;98:661–669. - PubMed
-
- Wilson JC, Furlano RI, Jick SS, Meier CR.. Inflammatory bowel disease and the risk of autoimmune diseases. J Crohns Colitis. 2016;10:186–193. - PubMed
-
- Xavier RJ, Podolsky DK.. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ.. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. - PubMed