Loss of Nexilin function leads to a recessive lethal fetal cardiomyopathy characterized by cardiomegaly and endocardial fibroelastosis
- PMID: 35166435
- PMCID: PMC9306924
- DOI: 10.1002/ajmg.a.62685
Loss of Nexilin function leads to a recessive lethal fetal cardiomyopathy characterized by cardiomegaly and endocardial fibroelastosis
Abstract
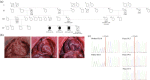
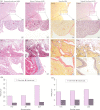
The Nexilin F-Actin Binding Protein (Nexilin) encoded by NEXN is a cardiac Z-disc protein important for cardiac function and development in humans, zebrafish, and mice. Heterozygote variants in the human NEXN gene have been reported to cause dilated and hypertrophic cardiomyopathy. Homozygous variants in NEXN cause a lethal form of human fetal cardiomyopathy, only described in two patients before. In a Swedish, four-generation, non-consanguineous family comprising 42 individuals, one female had three consecutive pregnancies with intrauterine fetal deaths caused by a lethal form of dilated cardiomyopathy. Whole-exome sequencing and variant analysis revealed that the affected fetuses were homozygous for a NEXN variant (NM_144573:c.1302del;p.(Ile435Serfs*3)). Moreover, autopsy and histology staining declared that they presented with cardiomegaly and endocardial fibroelastosis. Immunohistochemistry staining for Nexilin in the affected fetuses revealed reduced antibody staining and loss of striation in the heart, supporting loss of Nexilin function. Clinical examination of seven heterozygote carriers confirmed dilated cardiomyopathy (two individuals), other cardiac findings (three individuals), or no cardiac deviations (two individuals), indicating incomplete penetrance or age-dependent expression of dilated cardiomyopathy. RNA sequencing spanning the variant in cDNA blood of heterozygote individuals revealed nonsense-mediated mRNA decay of the mutated transcripts. In the current study, we present the first natural course of the recessively inherited lethal form of human fetal cardiomyopathy caused by loss of Nexilin function. The affected family had uneventful pregnancies until week 23-24, followed by fetal death at week 24-30, characterized by cardiomegaly and endocardial fibroelastosis.
Keywords: NEXN; Nexilin; cardiomyopathy; lethal.
© 2022 The Authors. American Journal of Medical Genetics Part A published by Wiley Periodicals LLC.
Figures

Similar articles
-
Nexilin in cardiomyopathy: unveiling its diverse roles with special focus on endocardial fibroelastosis.Heart Fail Rev. 2024 Sep;29(5):1025-1037. doi: 10.1007/s10741-024-10416-8. Epub 2024 Jul 10. Heart Fail Rev. 2024. PMID: 38985384 Review.
-
Knock-out of nexilin in mice leads to dilated cardiomyopathy and endomyocardial fibroelastosis.Basic Res Cardiol. 2016 Jan;111(1):6. doi: 10.1007/s00395-015-0522-5. Epub 2015 Dec 10. Basic Res Cardiol. 2016. PMID: 26659360
-
Childhood onset nexilin dilated cardiomyopathy: A heterozygous and a homozygous case.Am J Med Genet A. 2021 Aug;185(8):2464-2470. doi: 10.1002/ajmg.a.62231. Epub 2021 May 5. Am J Med Genet A. 2021. PMID: 33949776 Free PMC article. Review.
-
Biallelic NEXN variants and fetal onset dilated cardiomyopathy: two independent case reports and revision of literature.Ital J Pediatr. 2024 Aug 26;50(1):156. doi: 10.1186/s13052-024-01678-x. Ital J Pediatr. 2024. PMID: 39183344 Free PMC article. Review.
-
Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy.Nat Med. 2009 Nov;15(11):1281-8. doi: 10.1038/nm.2037. Epub 2009 Nov 1. Nat Med. 2009. PMID: 19881492
Cited by
-
Genetic Insights from Consanguineous Cardiomyopathy Families.Genes (Basel). 2023 Jan 10;14(1):182. doi: 10.3390/genes14010182. Genes (Basel). 2023. PMID: 36672924 Free PMC article.
-
NEXN deficiency leads to dilated cardiomyopathy in human pluripotent stem cell-derived cardiomyocytes.Stem Cell Res Ther. 2025 Jul 26;16(1):402. doi: 10.1186/s13287-025-04484-2. Stem Cell Res Ther. 2025. PMID: 40713745 Free PMC article.
-
The Role of Genetics in Risk Stratification Strategy of Dilated Cardiomyopathy.Rev Cardiovasc Med. 2022 Sep 9;23(9):305. doi: 10.31083/j.rcm2309305. eCollection 2022 Sep. Rev Cardiovasc Med. 2022. PMID: 39077708 Free PMC article. Review.
-
Unveiling the Spectrum of Minor Genes in Cardiomyopathies: A Narrative Review.Int J Mol Sci. 2024 Sep 10;25(18):9787. doi: 10.3390/ijms25189787. Int J Mol Sci. 2024. PMID: 39337275 Free PMC article. Review.
-
Two genetic variants in NEXN and ABCC6 genes found in a patient with right coronary artery to right ventricle fistula combined with giant coronary aneurysm and patent ductus arteriosus.Front Cardiovasc Med. 2022 Nov 17;9:1048795. doi: 10.3389/fcvm.2022.1048795. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36465446 Free PMC article.
References
-
- Aherrahrou, Z. , Schlossarek, S. , Stoelting, S. , Klinger, M. , Geertz, B. , Weinberger, F. , Kessler, T. , Aherrahrou, R. , Moreth, K. , Bekeredjian, R. , Hrabě De Angelis, M. , Just, S. , Rottbauer, W. , Eschenhagen, T. , Schunkert, H. , Carrier, L. , & Erdmann, J. (2016). Knock‐out of nexilin in mice leads to dilated cardiomyopathy and endomyocardial fibroelastosis. Basic Research in Cardiology, 111, 6. - PubMed
-
- Al‐Hassnan, Z. N. , Almesned, A. , Tulbah, S. , Al‐Manea, W. , & Al‐Fayyadh, M. (2013). Identification of a novel homozygous Nexn gene mutation in recessively inherited dilated cardiomyopathy. Journal of the Saudi Heart Association, 25, 171–172.
-
- Andersen, J. D. , Jacobsen, S. B. , Trudsø, L. C. , Kampmann, M. L. , Banner, J. , & Morling, N. (2019). Whole genome and transcriptome sequencing of post‐mortem cardiac tissues from sudden cardiac death victims identifies a gene regulatory variant in Nexn. International Journal of Legal Medicine, 133, 1699–1709. - PubMed
-
- Baig, M. K. , Goldman, J. H. , Caforio, A. L. , Coonar, A. S. , Keeling, P. J. , & Mckenna, W. J. (1998). Familial dilated cardiomyopathy: Cardiac abnormalities are common in asymptomatic relatives and may represent early disease. Journal of the American College of Cardiology, 31, 195–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

