Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features
- PMID: 35166597
- PMCID: PMC9221153
- DOI: 10.1089/nat.2021.0071
Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features
Abstract
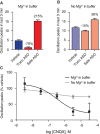
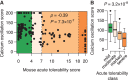
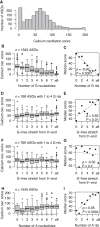
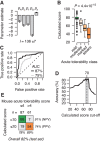
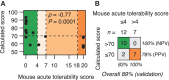
Antisense oligonucleotides are a relatively new therapeutic modality and safety evaluation is still a developing area of research. We have observed that some oligonucleotides can produce acute, nonhybridization dependent, neurobehavioral side effects after intracerebroventricular (ICV) dosing in mice. In this study, we use a combination of in vitro, in vivo, and bioinformatics approaches to identify a sequence design algorithm, which can reduce the number of acutely toxic molecules synthesized and tested in mice. We find a cellular assay measuring spontaneous calcium oscillations in neuronal cells can predict the behavioral side effects after ICV dosing, and may provide a mechanistic explanation for these observations. We identify sequence features that are overrepresented or underrepresented among oligonucleotides causing these reductions in calcium oscillations. A weighted linear combination of the five most informative sequence features predicts the outcome of ICV dosing with >80% accuracy. From this, we develop a bioinformatics tool that allows oligonucleotide designs with acceptable acute neurotoxic potential to be identified, thereby reducing the number of toxic molecules entering drug discovery pipelines. The informative sequence features we identified also suggest areas in which to focus future medicinal chemistry efforts.
Keywords: antisense oligonucleotides; calcium oscillations; intracerebroventricular dosing; neurotoxicity; sequence design algorithm.
Conflict of interest statement
P.H.H., A.M.H., and M.L.M. are employees of F. Hoffmann-La Roche, a company developing nucleic acid-based medicines. J.M.B., A.E., M.P., K.J., R.E.O., S.E.M., D.L., J.L., M.G., and A.M.C. are former or current employees of Bristol Myers Squibb and may hold company stock or stock options.
Figures

References
-
- Leavitt BR and Tabrizi SJ. (2020). Antisense oligonucleotides for neurodegeneration. Science 367:1428–1429. - PubMed
-
- Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, Iannaccone ST, Kirschner J, Kuntz NL, et al. (2018). Nusinersen versus Sham control in later-onset spinal muscular atrophy. N Engl J Med 378:625–635. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

