In Vitro Analysis of Nasal Interface Options for High-Efficiency Aerosol Administration to Preterm Infants
- PMID: 35166601
- PMCID: PMC9416545
- DOI: 10.1089/jamp.2021.0057
In Vitro Analysis of Nasal Interface Options for High-Efficiency Aerosol Administration to Preterm Infants
Abstract
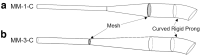
Background: An infant air-jet dry powder inhaler (DPI) platform has recently been developed that in combination with highly dispersible spray-dried powder formulations can achieve high-efficiency aerosolization with low actuation air volumes. The objective of this study was to investigate modifications to the nasal interface section of this platform to improve the aerosol delivery performance through preterm nose-throat (NT) models. Methods: Aerosol delivery performance of multiple nasal interface flow pathways and prong configurations was assessed with two in vitro preterm infant NT models. Two excipient-enhanced growth (EEG) dry powder formulations were explored containing either l-leucine or trileucine as the dispersion enhancer. Performance metrics included aerosol depositional loss in the nasal interface, deposition in the NT models, and tracheal filter deposition, which was used to estimate lung delivery efficiency. Results: The best performing nasal interface replaced the straight flexible prong of the original gradual expansion design with a rigid curved prong (∼20° curvature). The prong modification increased the lung delivery efficiency by 5%-10% (absolute difference) depending on the powder formulation. Adding a metal mesh to the flow pathway, to dissipate the turbulent jet, also improved lung delivery efficiency by ∼5%, while reducing the NT depositional loss by a factor of over twofold compared with the original nasal interface. The platform was also found to perform similarly in two different preterm NT models, with no statistically significant difference between any of the performance metrics. Conclusions: Modifications to the nasal interface of an infant air-jet DPI improved the aerosol delivery through multiple infant NT models, providing up to an additional 10% lung delivery efficiency (absolute difference) with the lead design delivering ∼57% of the loaded dose to the tracheal filter, while performance in two unique preterm airway geometries remained similar.
Keywords: air-jet DPI; high-dose DPI; infant DPI; nasal prong; nose-to-lung aerosol delivery; rapid aerosol administration; trans-nasal aerosol delivery.
Conflict of interest statement
Virginia Commonwealth University is currently pursuing patent protection of devices and methods described in this study, which if licensed and commercialized, may provide a future financial interest to the authors.
Figures




Similar articles
-
Development of an Infant Air-Jet Dry Powder Aerosol Delivery System (iDP-ADS) Including a New Multifunctional Bifurcating Two-Prong Nasal Interface.Pharm Res. 2025 Feb;42(2):365-384. doi: 10.1007/s11095-024-03814-y. Epub 2025 Feb 10. Pharm Res. 2025. PMID: 39930310 Free PMC article.
-
Characterizing the Effects of Nasal Prong Interfaces on Aerosol Deposition in a Preterm Infant Nasal Model.AAPS PharmSciTech. 2022 Apr 19;23(5):114. doi: 10.1208/s12249-022-02259-z. AAPS PharmSciTech. 2022. PMID: 35441324
-
Initial Development of an Air-Jet Dry Powder Inhaler for Rapid Delivery of Pharmaceutical Aerosols to Infants.J Aerosol Med Pulm Drug Deliv. 2021 Feb;34(1):57-70. doi: 10.1089/jamp.2020.1604. Epub 2020 Aug 4. J Aerosol Med Pulm Drug Deliv. 2021. PMID: 32758026 Free PMC article.
-
Physical stability of dry powder inhaler formulations.Expert Opin Drug Deliv. 2020 Jan;17(1):77-96. doi: 10.1080/17425247.2020.1702643. Epub 2019 Dec 13. Expert Opin Drug Deliv. 2020. PMID: 31815554 Free PMC article. Review.
-
Dry powder aerosol delivery systems: current and future research directions.J Aerosol Med. 2006 Spring;19(1):21-7. doi: 10.1089/jam.2006.19.21. J Aerosol Med. 2006. PMID: 16551211 Review.
Cited by
-
Development of a High-Dose Infant Air-Jet Dry Powder Inhaler (DPI) with Passive Cyclic Loading of the Formulation.Pharm Res. 2022 Dec;39(12):3317-3330. doi: 10.1007/s11095-022-03409-5. Epub 2022 Oct 17. Pharm Res. 2022. PMID: 36253630 Free PMC article.
-
Advancement of a high-dose infant air-jet dry powder inhaler (DPI) with passive cyclic loading: Performance tuning for different formulations.Int J Pharm. 2023 Aug 25;643:123199. doi: 10.1016/j.ijpharm.2023.123199. Epub 2023 Jul 4. Int J Pharm. 2023. PMID: 37406945 Free PMC article.
-
Development of an Infant Air-Jet Dry Powder Aerosol Delivery System (iDP-ADS) Including a New Multifunctional Bifurcating Two-Prong Nasal Interface.Pharm Res. 2025 Feb;42(2):365-384. doi: 10.1007/s11095-024-03814-y. Epub 2025 Feb 10. Pharm Res. 2025. PMID: 39930310 Free PMC article.
-
In Vitro Anatomical Models for Nasal Drug Delivery.Pharmaceutics. 2022 Jun 26;14(7):1353. doi: 10.3390/pharmaceutics14071353. Pharmaceutics. 2022. PMID: 35890249 Free PMC article. Review.
-
Aerosol Delivery of Lung Surfactant and Nasal CPAP in the Treatment of Neonatal Respiratory Distress Syndrome.Front Pediatr. 2022 Jun 15;10:923010. doi: 10.3389/fped.2022.923010. eCollection 2022. Front Pediatr. 2022. PMID: 35783301 Free PMC article. Review.
References
-
- Bhashyam AR, Wolf MT, Marcinkowski AL, Saville A, Thomas K, Carcillo JA, and Corcoran TE: Aerosol delivery through nasal cannulas: an in vitro study. J Aerosol Med Pulm Drug Deliv. 2008;21:181–187. - PubMed
-
- Fink JB: Delivery of inhaled drugs for infants and small children: a commentary on present and future needs. Clin Ther. 2012;34:S36–S45. - PubMed
-
- Corcoran TE, Saville A, Adams PS, Johnston DJ, Czachowski MR, Domnina YA, Lin JH, Weiner DJ, Huber AS, and Sanchez De Toledo J: Deposition studies of aerosol delivery by nasal cannula to infants. Pediatr Pulmonol. 2019;54:1319–1325. - PubMed
-
- Sunbul FS, Fink JB, Harwood R, Sheard MM, Zimmerman RD, and Ari A: Comparison of HFNC, bubble CPAP and SiPAP on aerosol delivery in neonates: an in-vitro study. Pediatr Pulmonol. 2015;50:1099–1106. - PubMed
-
- El Taoum KK, Xi J, Kim J, and Berlinski A: In vitro evaluation of aerosols delivered via the nasal route. Respir Care. 2015;60:1015–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

