Association of Adjuvant Hormone Therapy Timing With Overall Survival Among Patients With Hormone Receptor-Positive Human Epidermal Growth Factor Receptor-2-Negative Early Breast Cancer Without Chemotherapy
- PMID: 35166783
- PMCID: PMC8848199
- DOI: 10.1001/jamanetworkopen.2021.45934
Association of Adjuvant Hormone Therapy Timing With Overall Survival Among Patients With Hormone Receptor-Positive Human Epidermal Growth Factor Receptor-2-Negative Early Breast Cancer Without Chemotherapy
Abstract
Importance: Studies have shown that delayed initiation of surgery and adjuvant chemotherapy is associated with lower rates of breast cancer survival. However, it remains unclear whether delayed initiation of adjuvant hormone therapy (AHT) is associated with survival.
Objective: To assess the association of time to adjuvant hormone therapy (TTH) with breast cancer survival and evaluate the factors associated with AHT.
Design, setting, and participants: This cohort study examined data from the National Cancer Database from 2004 through 2014 to assess the association of TTH (stratified as ≤150 and >150 days) with cancer survival. All patients included were diagnosed with stage I to stage III hormone receptor-positive, human epidermal growth factor receptor-2 (ERBB2; formerly HER2)-negative invasive breast cancer and underwent AHT without chemotherapy. Data were analyzed from April 2019 to May 2020.
Exposures: AHT was administered at different time points following surgical procedures for breast cancer treatment.
Main outcomes and measures: An inverse probability of treatment weighting (IPTW) model was constructed to evaluate overall survival by adjusting for treatment facility, patient demographics, tumor characteristics, and treatment; multivariable logistic regression was conducted to assess factors associated with delayed treatment.
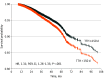
Results: A total of 144 103 patients (median [IQR] follow-up, 36.6 months [25.5-49.2 months]; mean [SD] age, 63.7 [11.6] years) were identified, which included 142 916 (99.2%) women, 11 574 (8.0%) Black patients, and 126 013 (87.4%) White patients. Of these, 134 873 patients (93.6%) had a TTH of 150 days or less and 9230 patients (6.4%) had a TTH longer than 150 days. The IPTW-based Cox model demonstrated that patients with delayed AHT (ie, a TTH past 150 days) were associated with decreased survival (hazard ratio [HR], 1.31; 95% CI, 1.26-1.35; P < .001) compared with those receiving the timely treatment (TTH ≤150 days). Several sensitivity analyses (including IPTW with stabilized weight [HR, 1.31; 95% CI, 1.19-1.45; P < .001], propensity score matching [HR, 1.41; 1.13-1.76; P = .002], and propensity score regression adjustment [HR, 1.29; 95% CI, 1.16-1.43; P < .001]) and exploratory subgroup analyses yielded similar trends. Factors associated with delayed AHT included Black racial identity (OR, 1.66; 95% CI, 1.55-1.77), nonprivate insurance (eg, no insurance: OR, 1.46; 95% CI, 1.26-1.70), living in large metropolitan or metropolitan areas (reference vs urban, less urban, or rural: OR, 0.82; 95% CI, 0.76-0.87), treatment in a community hospital (reference vs academic or research: OR, 0.91; 95% CI, 0.84-0.98), Charlson-Deyo Comorbidity Index score 2 or higher (OR, 1.17; 95% CI, 1.04-1.32), poor grade differentiation (OR, 1.42; 95% CI, 1.32-1.53), II and III pathological stage (stage III: OR, 3.13; 95% CI, 2.76-3.54), estrogen receptor-positive (ER+)/progesterone receptor-negative (PR-) or ER-/PR+ (OR, 1.22; 95% CI, 1.13-1.31), receiving breast conservation surgery (reference vs mastectomy: OR, 0.87; 95% CI, 0.79-0.94), and radiotherapy (reference vs no radiotherapy: OR, 0.56; 95% CI, 0.52-0.61).
Conclusions and relevance: The delay of the initiation of AHT past 150 days was associated with diminished survival in hormone receptor-positive, ERBB2-negative patients with breast cancer who did not receive chemotherapy. Efforts should be made to address factors associated with delayed treatment to improve survival.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

