Integrity of the Actin Cytoskeleton of Host Macrophages is Necessary for Mycobacterial Entry
- PMID: 35166859
- PMCID: PMC8852914
- DOI: 10.1007/s00232-022-00217-1
Integrity of the Actin Cytoskeleton of Host Macrophages is Necessary for Mycobacterial Entry
Abstract
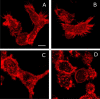
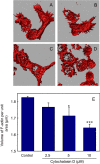
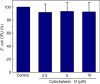
Macrophages are the primary hosts for Mycobacterium tuberculosis (M. tb), an intracellular pathogen, and the causative organism of tuberculosis (TB) in humans. While M. tb has the ability to enter and survive in host macrophages, the precise mechanism of its internalization, and factors that control this essential process are poorly defined. We have previously demonstrated that perturbations in levels of cholesterol and sphingolipids in macrophages lead to significant reduction in the entry of Mycobacterium smegmatis (M. smegmatis), a surrogate model for mycobacterial internalization, signifying a role for these plasma membrane lipids in interactions at the host-pathogen interface. In this work, we investigated the role of the host actin cytoskeleton, a critical protein framework underlying the plasma membrane, in the entry of M. smegmatis into human macrophages. Our results show that cytochalasin D mediated destabilization of the actin cytoskeleton of host macrophages results in a dose-dependent reduction in the entry of mycobacteria. Notably, the internalization of Escherichia coli remained invariant upon actin destabilization of host cells, implying a specific involvement of the actin cytoskeleton in mycobacterial infection. By monitoring the F-actin content of macrophages utilizing a quantitative confocal microscopy-based technique, we observed a close correlation between the entry of mycobacteria into host macrophages with cellular F-actin content. Our results constitute the first quantitative analysis of the role of the actin cytoskeleton of human macrophages in the entry of mycobacteria, and highlight actin-mediated mycobacterial entry as a potential target for future anti-TB therapeutics.
Keywords: Actin cytoskeleton; Cytochalasin D; F-actin quantitation; Macrophages; Mycobacterium.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
Integrity of the actin cytoskeleton of host macrophages is essential for Leishmania donovani infection.Biochim Biophys Acta. 2014 Aug;1838(8):2011-8. doi: 10.1016/j.bbamem.2014.04.017. Epub 2014 Apr 26. Biochim Biophys Acta. 2014. PMID: 24780377
-
Macrophage sphingolipids are essential for the entry of mycobacteria.Chem Phys Lipids. 2018 Jul;213:25-31. doi: 10.1016/j.chemphyslip.2018.03.004. Epub 2018 Mar 8. Chem Phys Lipids. 2018. PMID: 29526700
-
Dissecting the membrane cholesterol requirement for mycobacterial entry into host cells.Chem Phys Lipids. 2015 Jul;189:19-27. doi: 10.1016/j.chemphyslip.2015.05.006. Epub 2015 May 26. Chem Phys Lipids. 2015. PMID: 26021693
-
Quantitation of F-actin in cytoskeletal reorganization: Context, methodology and implications.Methods. 2024 Oct;230:44-58. doi: 10.1016/j.ymeth.2024.07.009. Epub 2024 Jul 27. Methods. 2024. PMID: 39074540 Review.
-
Cell death at the cross roads of host-pathogen interaction in Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2018 Dec;113:99-121. doi: 10.1016/j.tube.2018.09.007. Epub 2018 Sep 29. Tuberculosis (Edinb). 2018. PMID: 30514519 Review.
Cited by
-
Statin-induced increase in actin polymerization modulates GPCR dynamics and compartmentalization.Biophys J. 2023 Jun 6;122(11):1938-1955. doi: 10.1016/j.bpj.2022.08.039. Epub 2022 Aug 30. Biophys J. 2023. PMID: 36045572 Free PMC article.
-
Editorial: Vesicular transport, the actin cytoskeleton and their involvement in virulence mechanisms during host-parasite interaction.Front Cell Infect Microbiol. 2023 Jun 14;13:1229067. doi: 10.3389/fcimb.2023.1229067. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37389219 Free PMC article. No abstract available.
-
Hypoxia Modulates Membrane Mechanics in Pancreatic Cancer.J Membr Biol. 2025 Aug 18. doi: 10.1007/s00232-025-00358-z. Online ahead of print. J Membr Biol. 2025. PMID: 40824545
-
Exploring host-pathogen interactions in the Dictyostelium discoideum-Mycobacterium marinum infection model of tuberculosis.Dis Model Mech. 2024 Jul 1;17(7):dmm050698. doi: 10.1242/dmm.050698. Epub 2024 Jul 22. Dis Model Mech. 2024. PMID: 39037280 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

