Population Pharmacokinetic Model for Tramadol and O-desmethyltramadol in Older Patients
- PMID: 35167052
- PMCID: PMC9050769
- DOI: 10.1007/s13318-022-00756-x
Population Pharmacokinetic Model for Tramadol and O-desmethyltramadol in Older Patients
Abstract
Background and objectives: Tramadol is commonly prescribed to manage chronic pain in older patients. However, there is a gap in the literature describing the pharmacokinetic parameters for tramadol and its active metabolite (O-desmethyltramadol [ODT]) in this population. The objective of this study was to develop and evaluate a population pharmacokinetic model for tramadol and ODT in older patients.
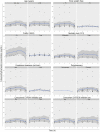
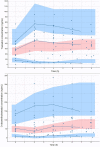
Methods: Twenty-one patients who received an extended-release oral tramadol dose (25-100 mg) were recruited. Tramadol and ODT concentrations were determined using a validated liquid chromatography/tandem mass spectrometry method. A population pharmacokinetic model was developed using non-linear mixed-effects modelling. The performance of the model was assessed by visual predictive check.
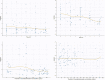
Results: A two-compartment, first-order absorption model with linear elimination best described the tramadol concentration data. The absorption rate constant was 2.96/h (between-subject variability [BSV] 37.8%), apparent volume of distribution for the central compartment (V1/F) was 0.373 l (73.8%), apparent volume of distribution for the peripheral compartment (V2/F) was 0.379 l (97.4%), inter-compartmental clearance (Q) was 0.0426 l/h (2.19%) and apparent clearance (CL/F) was 0.00604 l/h (6.61%). The apparent rate of metabolism of tramadol to ODT (kt) was 0.0492 l/h (78.5%) and apparent clearance for ODT (CLm) was 0.143 l/h (21.6%). Identification of Seniors at Risk score (ISAR) and creatinine clearance (CrCL) were the only covariates included in the final model, where a higher value for the ISAR increased the maximum concentration (Cmax) of tramadol and reduced the BSV in Q from 4.71 to 2.19%. A higher value of CrCL reduced tramadol Cmax and half-life (T1/2) and reduced the BSV in V2/F (from 148 to 97.4%) and in CL/F (from 78.9 to 6.61%).
Conclusion: Exposure to tramadol increased with increased frailty and reduced CrCL. Prescribers should consider patients frailty status and CrCL to minimise the risk of tramadol toxicity in such cohort of patients.
© 2022. The Author(s).
Conflict of interest statement
The authors have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Figures








References
-
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an’atypical’opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

