Rv0500A is a transcription factor that links Mycobacterium tuberculosis environmental response with division and impacts host colonization
- PMID: 35167150
- PMCID: PMC9382876
- DOI: 10.1111/mmi.14886
Rv0500A is a transcription factor that links Mycobacterium tuberculosis environmental response with division and impacts host colonization
Abstract
For Mycobacterium tuberculosis (Mtb) to successfully infect a host, it must be able to adapt to changes in its microenvironment, including variations in ionic signals such as pH and chloride (Cl- ), and link these responses to its growth. Transcriptional changes are a key mechanism for Mtb environmental adaptation, and we identify here Rv0500A as a novel transcriptional regulator that links Mtb environmental response and division processes. Global transcriptional profiling revealed that Rv0500A acts as a repressor and influences the expression of genes related to division, with the magnitude of its effect modulated by pH and Cl- . Rv0500A can directly bind the promoters of several of these target genes, and we identify key residues required for its DNA-binding ability and biological effect. Overexpression of rv0500A disrupted Mtb growth morphology, resulting in filamentation that was exacerbated by high environmental Cl- levels and acidic pH. Finally, we show that perturbation of rv0500A leads to attenuation of the ability of Mtb to colonize its host in vivo. Our work highlights the important link between Mtb environmental response and growth characteristics, and uncovers a new transcription factor involved in this critical facet of Mtb biology.
Keywords: Mycobacterium tuberculosis; chloride; gene expression regulation; host-pathogen interactions; hydrogen ion concentration; transcription factor.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists.
Figures



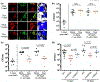
References
-
- Aguilar D, Infante E, Martin C, Gormley E, Gicquel B & Hernandez Pando R (2007) Immunological responses and protective immunity against tuberculosis conferred by vaccination of Balb/C mice with the attenuated mycobacterium tuberculosis (phoP) SO2 strain. Clinical and Experimental Immunology, 147, 330–338. - PMC - PubMed
-
- Asensio JG, Arbues A, Marinova D & Martin C (2010) A new live tuberculosis vaccine based on phoP inactivation. Revista Portuguesa de Pneumologia, 16sa, S43–S48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

