Cryptic MYC insertions in Burkitt lymphoma: New data and a review of the literature
- PMID: 35167621
- PMCID: PMC8846522
- DOI: 10.1371/journal.pone.0263980
Cryptic MYC insertions in Burkitt lymphoma: New data and a review of the literature
Abstract
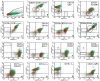
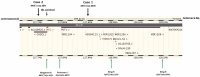
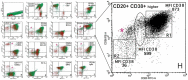
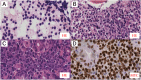
The occurrence of MYC-negative Burkitt lymphoma (BL) has been discussed for many years. The real frequency of the MYC insertion in MYC-negative BL is still unknown. Fine-needle aspiration biopsies of 108 consecutive patients with clinicopathologically suspected BL (suspBL) were evaluated by flow cytometry, classical cytogenetics, and fluorescence in situ hybridization (FISH). We found 12 cases (11%) without the MYC rearrangement by FISH with a MYC breakapart probe: two patients (1.9%) with cryptic MYC/IGH fusion (finally diagnosed as BL) and 10 patients (9.3%) with 11q gain/loss (finally diagnosed as Burkitt-like lymphoma with 11q aberration). The exact breakpoints of the cryptic MYC/IGH were investigated by next-generation sequencing. The MYC insertions' breakpoints were identified in PVT1 in the first case, and 42 kb upstream of 5'MYC in the second case. To date, a molecular characterization of the MYC insertion in BL has only been reported in one case. Detailed descriptions of our MYC insertions in a routinely and consecutively diagnosed suspBL cohort will contribute to resolving the issue of MYC negativity in BL. In our opinion, the presence of the MYC insertions in BL and other lymphomas might be underestimated, because routine genetic diagnostics are usually based on FISH only, without karyotyping.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bertrand S, Berger R, Philip T, Bernheim A, Bryon PA, Bertoglio J, et al.. Variant translocation in a non-endemic case of Burkitt’s lymphoma: t(8;22) in an Epstein-Barr virus-negative tumour and in a derived cell line. Eur J Cancer. 1981; 17: 577–584. doi: 10.1016/0014-2964(81)90060-8 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

