Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial
- PMID: 35167655
- PMCID: PMC9012129
- DOI: 10.1182/blood.2021014738
Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial
Abstract
CD19-directed chimerical antigen receptor T-cell (CAR-T) products have gained US Food and Drug Administration approval for systemic large B-cell lymphoma. Because of concerns about potential immune cell-associated neurotoxicity syndrome (ICANS), patients with primary central nervous system (CNS) lymphoma (PCNSL) were excluded from all pivotal CAR-T studies. We conducted a phase 1/2 clinical trial of tisagenlecleucel in a highly refractory patients with PCNSL and significant unmet medical need. Here, we present results of 12 relapsed patients with PCNSL who were treated with tisagenlecleucel and followed for a median time of 12.2 months (range, 3.64-23.5). Grade 1 cytokine release syndrome was observed in 7/12 patients (58.3%), low-grade ICANS in 5/12 (41.6%) patients, and only 1 patient experienced grade 3 ICANS. Seven of 12 patients (58.3%) demonstrated response, including a complete response in 6/12 patients (50%). There were no treatment-related deaths. Three patients had ongoing complete remission at data cutoff. Tisagenlecleucel expanded in the peripheral blood and trafficked to the CNS. Exploratory analysis identified T-cell, CAR T, and macrophage gene signatures in cerebrospinal fluid following infusion when compared with baseline. Overall, tisagenlecleucel was well tolerated and resulted in a sustained remission in 3/7 (42.9%) of initial responders. These data suggest that tisagenlecleucel is safe and effective in this highly refractory patient population. This trial was registered at www.clinicaltrials.gov as #NCT02445248.
© 2022 by The American Society of Hematology.
Figures
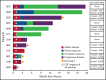
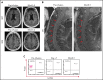
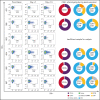


Comment in
-
No CNS sanctuary for lymphoma from CAR T.Blood. 2022 Apr 14;139(15):2261-2263. doi: 10.1182/blood.2022015802. Blood. 2022. PMID: 35420689 No abstract available.
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. - PubMed
-
- Batchelor TT, DeAngelis LM. Lymphoma and Leukemia of the Nervous System, 2nd ed. New York, NY: Springer; 2013.
-
- Green K, Hogg JP. Central Nervous System Lymphoma. Treasure Island, FL: StatPearls; 2021. - PubMed

