It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease
- PMID: 35167695
- PMCID: PMC9359450
- DOI: 10.1210/clinem/dgac045
It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease
Abstract
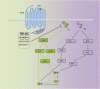
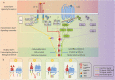
Context: Thyroid eye disease (TED) is a complex autoimmune disease process. Orbital fibroblasts represent the central orbital immune target. Involvement of the TSH receptor (TSHR) in TED is not fully understood. IGF-I receptor (IGF-IR) is overexpressed in several cell types in TED, including fibrocytes and orbital fibroblasts. IGF-IR may form a physical and functional complex with TSHR.
Objective: Review literature relevant to autoantibody generation in TED and whether these induce orbital fibroblast responses directly through TSHR, IGF-IR, or both.
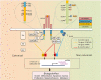
Evidence: IGF-IR has traditionally been considered a typical tyrosine kinase receptor in which tyrosine residues become phosphorylated following IGF-I binding. Evidence has emerged that IGF-IR possesses kinase-independent activities and can be considered a functional receptor tyrosine kinase/G-protein-coupled receptor hybrid, using the G-protein receptor kinase/β-arrestin system. Teprotumumab, a monoclonal IGF-IR antibody, effectively reduces TED disease activity, proptosis, and diplopia. In addition, the drug attenuates in vitro actions of both IGF-I and TSH in fibrocytes and orbital fibroblasts, including induction of proinflammatory cytokines by TSH and TED IgGs.
Conclusions: Although teprotumumab has been proven effective and relatively safe in the treatment of TED, many questions remain pertaining to IGF-IR, its relationship with TSHR, and how the drug might be disrupting these receptor protein/protein interactions. Here, we propose 4 possible IGF-IR activation models that could underlie clinical responses to teprotumumab observed in patients with TED. Teprotumumab is associated with several adverse events, including hyperglycemia and hearing abnormalities. Underpinning mechanisms of these are being investigated. Patients undergoing treatment with drug must be monitored for these and managed with best medical practices.
Keywords: GPCR; IGF-I; IGF-IR; IGF1; IGF1R; RTK; TSHR; beta-arrestins; biased signaling.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Watt T, Cramon P, Hegedüs L, et al. The thyroid-related quality of life measure ThyPRO has good responsiveness and ability to detect relevant treatment effects. J Clin Endocrinol Metab. 2014;99(10):3708-3717. - PubMed
-
- Bartalena L, Kahaly GJ, Baldeschi L, et al. ; EUGOGO † . The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185(4):G43-G67. - PubMed
-
- Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1565. - PubMed
-
- Chin YH, Ng CH, Lee MH, et al. Prevalence of thyroid eye disease in Graves’ disease: a meta-analysis and systematic review. Clin Endocrinol (Oxf). 2020;93(4):363-374. - PubMed
-
- Leo M, Menconi F, Rocchi R, et al. Role of the underlying thyroid disease on the phenotype of Graves’ orbitopathy in a tertiary referral center. Thyroid. 2015;25(3):347-351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

