Molecular Mechanisms Underlying Neurotransmitter Release
- PMID: 35167762
- PMCID: PMC9490555
- DOI: 10.1146/annurev-biophys-111821-104732
Molecular Mechanisms Underlying Neurotransmitter Release
Abstract
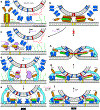
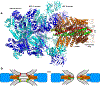
Major recent advances and previous data have led to a plausible model of how key proteins mediate neurotransmitter release. In this model, the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) proteins syntaxin-1, SNAP-25, and synaptobrevin form tight complexes that bring the membranes together and are crucial for membrane fusion. NSF and SNAPs disassemble SNARE complexes and ensure that fusion occurs through an exquisitely regulated pathway that starts with Munc18-1 bound to a closed conformation of syntaxin-1. Munc18-1 also binds to synaptobrevin, forming a template to assemble the SNARE complex when Munc13-1 opens syntaxin-1 while bridging the vesicle and plasma membranes. Synaptotagmin-1 and complexin bind to partially assembled SNARE complexes, likely stabilizing them and preventing fusion until Ca2+ binding to synaptotagmin-1 causes dissociation from the SNARE complex and induces interactions with phospholipids that help trigger release. Although fundamental questions remain about the mechanism of membrane fusion, these advances provide a framework to investigate the mechanisms underlying presynaptic plasticity.
Keywords: Munc13; Munc18; NSF; SNAPs; SNAREs; complexin; membrane fusion; neurotransmitter release; synaptotagmin.
Figures



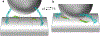

References
-
- Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, et al. 2006. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol 13: 209–17 - PubMed
-
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. 1999. Drosophila UNC-13 is essential for synaptic transmission. Nat. Neurosci 2: 965–71 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

