Lyme disease transmission by severely impaired ticks
- PMID: 35167765
- PMCID: PMC8846998
- DOI: 10.1098/rsob.210244
Lyme disease transmission by severely impaired ticks
Abstract
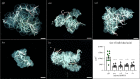
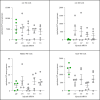
It has been demonstrated that impairing protein synthesis using drugs targeted against tRNA amino acid synthetases presents a promising strategy for the treatment of a wide variety of parasitic diseases, including malaria and toxoplasmosis. This is the first study evaluating tRNA synthetases as potential drug targets in ticks. RNAi knock-down of all tested tRNA synthetases had a strong deleterious phenotype on Ixodes ricinus feeding. Our data indicate that tRNA synthetases represent attractive, anti-tick targets warranting the design of selective inhibitors. Further, we tested whether these severely impaired ticks were capable of transmitting Borrelia afzelii spirochaetes. Interestingly, biologically handicapped I. ricinus nymphs transmitted B. afzelii in a manner quantitatively sufficient to develop a systemic infection in mice. These data suggest that initial blood-feeding, despite the incapability of ticks to fully feed and salivate, is sufficient for activating B. afzelii from a dormant to an infectious mode, enabling transmission and dissemination in host tissues.
Keywords: Borrelia; Lyme disease; borreliosis; tRNA synthetase; tick; transmission.
Conflict of interest statement
The authors declare no conflicts of interest associated with this manuscript.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
