Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study
- PMID: 35168079
- PMCID: PMC8813598
- DOI: 10.1016/j.intimp.2022.108570
Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study
Abstract
Background: Regdanvimab (CT-P59) is a neutralizing antibody authorized in Republic of Korea for the treatment of adult patients with moderate or mild-COVID-19 who are not on supplemental oxygen and have high risk of progressing to severe disease (age ≥ 50 years or comorbidities). This study evaluated the clinical efficacy, safety and medical utilization/costs associated with real-world regdanvimab therapy.
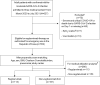
Methods: This non-interventional, retrospective cohort study included adult patients with confirmed mild-to-moderate SARS-CoV-2 infection. Patients treated with regdanvimab were compared with controls who had received other therapies. The primary endpoint was the proportion of patients progressing to severe/critical COVID-19 or death due to SARS-CoV-2 infection up to Day 28. Propensity score matching was applied to efficacy analyses.
Results: Overall, 552 patients were included in the Safety and Efficacy Sets (regdanvimab, n = 156; control, n = 396) and 274 patients in the propensity score-matched (PSM) Efficacy Set (regdanvimab, n = 113; control, n = 161). In the PSM Set, the risk of severe/critical COVID-19 or death was significantly lower in the regdanvimab group (7.1% vs 16.1%, P = 0.0263); supplemental oxygen was required by 8.0% and 18.6% of patients in the regdanvimab and control groups, respectively (P = 0.0128). There were no unexpected safety findings in the regdanvimab group. Medical utilization analysis showed an overall cost reduction with regdanvimab compared with control treatments.
Conclusions: Regdanvimab significantly reduced the proportion of patients progressing to severe/critical disease or dying of SARS-CoV-2 infection. This study shows the potential benefits of regdanvimab in reducing disease severity and improving medical utility in patients with COVID-19.
Keywords: COVID-19; CT-P59; Outcome; Pneumonia.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
SWL, SOL and JEL have been an investigator for clinical trials sponsored by Daewoong Pharmaceutical Co., Ltd., Pfizer, Chong Kun Dang Pharmaceutical and Shin Poong Pharmaceutical Co., Ltd., outside the scope of the submitted work.
K-HK has been an investigator for clinical trials sponsored by SD Biosensor, Inc., Abbott and Kogene Biotech Co., Ltd, outside the scope of the submitted work.
SHL has been an investigator for clinical trials sponsored by GlaxoSmithKline plc., Gilead Sciences, and Merck Sharp & Dohme Corp., outside the scope of the submitted work.
SYH and KTK have been investigators for clinical trials sponsored by Pfizer, Chong Kun Dang Pharmaceutical, SK Bioscience Co., Ltd. and Celltrion, Inc., outside the scope of the submitted work.
SW-K, YJK and SHB have been investigators for clinical trials sponsored by Dong Wha Pharmaceutical Co., Ltd., Daewoong Pharmaceutical Co., Ltd., ImmunMed, Inc., Green Cross Corporation, Shin Poong Pharmaceutical Co., Ltd. and Gilead Sciences, outside the scope of the submitted work.
H-HC has been an investigators for clinical trials sponsored by Green Cross Corporation, Celltrion, Inc., Shin Poong Parmaceutical Co., Ltd., and Gilead Sciences, outside the scope of the submitted work and also reports consulting fees from Green Cross Corporation.
A-SK declares no conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- World Health Organization, WHO coronavirus disease (COVID-19) dashboard, 2021. https://covid19.who.int/ (accessed 27 June 2021).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

