Biomarker identification using dynamic time warping analysis: a longitudinal cohort study of patients with COVID-19 in a UK tertiary hospital
- PMID: 35168965
- PMCID: PMC8852240
- DOI: 10.1136/bmjopen-2021-050331
Biomarker identification using dynamic time warping analysis: a longitudinal cohort study of patients with COVID-19 in a UK tertiary hospital
Abstract
Objectives: COVID-19 is a heterogeneous disease, and many reports have described variations in demographic, biochemical and clinical features at presentation influencing overall hospital mortality. However, there is little information regarding longitudinal changes in laboratory prognostic variables in relation to disease progression in hospitalised patients with COVID-19.
Design and setting: This retrospective observational report describes disease progression from symptom onset, to admission to hospital, clinical response and discharge/death among patients with COVID-19 at a tertiary centre in South East England.
Participants: Six hundred and fifty-one patients treated for SARS-CoV-2 between March and September 2020 were included in this analysis. Ethical approval was obtained from the HRA Specific Review Board (REC 20/HRA/2986) for waiver of informed consent.
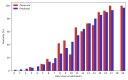
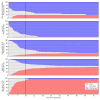
Results: The majority of patients presented within 1 week of symptom onset. The lowest risk patients had low mortality (1/45, 2%), and most were discharged within 1 week after admission (30/45, 67%). The highest risk patients, as determined by the 4C mortality score predictor, had high mortality (27/29, 93%), with most dying within 1 week after admission (22/29, 76%). Consistent with previous reports, most patients presented with high levels of C reactive protein (CRP) (67% of patients >50 mg/L), D-dimer (98%>upper limit of normal (ULN)), ferritin (65%>ULN), lactate dehydrogenase (90%>ULN) and low lymphocyte counts (81%<lower limit of normal (LLN)). Increases in platelet counts and decreases in CRP, neutrophil:lymphocyte ratio (p<0.001), lactate dehydrogenase, neutrophil counts, urea and white cell counts (all p<0.01) were each associated with discharge.
Conclusions: Serial measurement of routine blood tests may be a useful prognostic tool for monitoring treatment response in hospitalised patients with COVID-19. Changes in other biochemical parameters often included in a 'COVID-19 bundle' did not show significant association with outcome, suggesting there may be limited clinical benefit of serial sampling. This may have direct clinical utility in the context of escalating healthcare costs of the pandemic.
Keywords: COVID-19; respiratory infections; respiratory medicine (see thoracic medicine).
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
