The German Gestational Diabetes Study (PREG), a prospective multicentre cohort study: rationale, methodology and design
- PMID: 35168986
- PMCID: PMC8852757
- DOI: 10.1136/bmjopen-2021-058268
The German Gestational Diabetes Study (PREG), a prospective multicentre cohort study: rationale, methodology and design
Abstract
Introduction: Even well-treated gestational diabetes mellitus (GDM) might still have impact on long-term health of the mother and her offspring, although this relationship has not yet been conclusively studied. Using in-depth phenotyping of the mother and her offspring, we aim to elucidate the relationship of maternal hyperglycaemia during pregnancy and adequate treatment, and its impact on the long-term health of both mother and child.
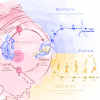
Methods: The multicentre PREG study, a prospective cohort study, is designed to metabolically and phenotypically characterise women with a 75-g five-point oral glucose tolerance test (OGTT) during, and repeatedly after pregnancy. Outcome measures are maternal glycaemia during OGTTs, birth outcome and the health and growth development of the offspring. The children of the study participants are followed up until adulthood with developmental tests and metabolic and epigenetic phenotyping in the PREG Offspring study. A total of 800 women (600 with GDM, 200 controls) will be recruited.
Ethics and dissemination: The study protocol has been approved by all local ethics committees. Results will be disseminated via conference presentations and peer-reviewed publications.
Trial registration number: The PREG study and the PREG Offspring study are registered with Clinical Trials (ClinicalTrials.gov identifiers: NCT04270578, NCT04722900).
Keywords: diabetes in pregnancy; maternal medicine; public health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RW reports lecture fees from Novo Nordisk and travel grants from Eli Lilly. He served on the advisory board of Akcea Therapeutics. In addition to his current work, ALB reports lecture fees from Astra Zeneca, Boehringer Ingelheim and NovoNordisk. He also served on advisory boards of Astra Zeneca, Boehringer Ingelheim and Novo Nordisk. Besides his current work, AF reports lecture fees and advisory board membership from Sanofi, Novo Nordisk, Eli Lilly and Astra Zeneca. In addition to his current work, MH reports research grants from Boehringer Ingelheim and Sanofi (both to the University Hospital of Tübingen) and lecture fees from Amryt, Sanofi, Novo Nordisk, Eli Lilly and Merck Sharp Dohme. He served on an advisory board for Boehringer Ingelheim. MR serves on advisory board and/or received lecture fees from Boehringer-Ingelheim Pharma, Eli Lilly, Fishawack Group, Novo Nordisk, Sanofi US, Target NASH and Terra Firma, as well as investigator-initiated research support from Boehringer-Ingelheim, Nutricia/Danone and Sanofi-Aventis.
References
-
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:e98–82. 10.2337/dc10-0719 - DOI - PubMed
-
- Duran A, Sáenz S, Torrejón MJ, et al. . Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos gestational diabetes study. Diabetes Care 2014;37:2442–50. 10.2337/dc14-0179 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical