AMIGO1 Promotes Axon Growth and Territory Matching in the Retina
- PMID: 35169021
- PMCID: PMC8973419
- DOI: 10.1523/JNEUROSCI.1164-21.2022
AMIGO1 Promotes Axon Growth and Territory Matching in the Retina
Abstract
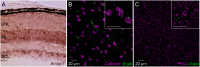
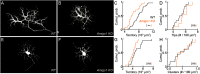
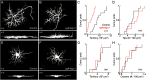
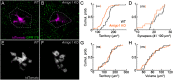
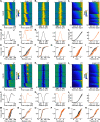
Dendrite and axon arbor sizes are critical to neuronal function and vary widely between different neuron types. The relative dendrite and axon sizes of synaptic partners control signal convergence and divergence in neural circuits. The developmental mechanisms that determine cell-type-specific dendrite and axon size and match synaptic partners' arbor territories remain obscure. Here, we discover that retinal horizontal cells express the leucine-rich repeat domain cell adhesion molecule AMIGO1. Horizontal cells provide pathway-specific feedback to photoreceptors-horizontal cell axons to rods and horizontal cell dendrites to cones. AMIGO1 selectively expands the size of horizontal cell axons. When Amigo1 is deleted in all or individual horizontal cells of either sex, their axon arbors shrink. By contrast, horizontal cell dendrites and synapse formation of horizontal cell axons and dendrites are unaffected by AMIGO1 removal. The dendrites of rod bipolar cells, which do not express AMIGO1, shrink in parallel with horizontal cell axons in Amigo1 knockout (Amigo1 KO) mice. This territory matching maintains the function of the rod bipolar pathway, preserving bipolar cell responses and retinal output signals in Amigo1 KO mice. We previously identified AMIGO2 as a scaling factor that constrains retinal neurite arbors. Our current results identify AMIGO1 as a scaling factor that expands retinal neurite arbors and reveal territory matching as a novel homeostatic mechanism. Territory matching interacts with other homeostatic mechanisms to stabilize the development of the rod bipolar pathway, which mediates vision near the threshold.SIGNIFICANCE STATEMENT Neurons send and receive signals through branched axonal and dendritic arbors. The size of these arbors is critical to the function of a neuron. Axons and dendrites grow during development and are stable at maturity. The mechanisms that determine axon and dendrite size are not well understood. Here, we identify a cell surface protein, AMIGO1, that selectively promotes axon growth of horizontal cells, a retinal interneuron. Removal of AMIGO1 reduces the size of horizontal cell axons without affecting the size of their dendrites or the ability of both arbors to form connections. The changes in horizontal cell axons are matched by changes in synaptic partner dendrites to stabilize retinal function. This identifies territory matching as a novel homeostatic plasticity mechanism.
Keywords: LRR protein; arbor size; circuit development; horizontal cell; rod bipolar pathway.
Copyright © 2022 the authors.
Figures



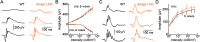
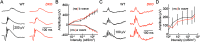

Similar articles
-
DSCAM-mediated control of dendritic and axonal arbor outgrowth enforces tiling and inhibits synaptic plasticity.Proc Natl Acad Sci U S A. 2017 Nov 21;114(47):E10224-E10233. doi: 10.1073/pnas.1713548114. Epub 2017 Nov 7. Proc Natl Acad Sci U S A. 2017. PMID: 29114051 Free PMC article.
-
Photoreceptor degeneration induces homeostatic rewiring of rod bipolar cells.Curr Biol. 2025 Jul 7;35(13):3263-3268.e2. doi: 10.1016/j.cub.2025.05.057. Epub 2025 Jun 25. Curr Biol. 2025. PMID: 40570846
-
Evolution of rod bipolar cells and rod vision.J Physiol. 2025 Jan 7. doi: 10.1113/JP287652. Online ahead of print. J Physiol. 2025. PMID: 39775947 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Behavioral interventions to reduce risk for sexual transmission of HIV among men who have sex with men.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD001230. doi: 10.1002/14651858.CD001230.pub2. Cochrane Database Syst Rev. 2008. PMID: 18646068
Cited by
-
Cellular and Molecular Mechanisms Regulating Retinal Synapse Development.Annu Rev Vis Sci. 2024 Sep;10(1):377-402. doi: 10.1146/annurev-vision-102122-105721. Annu Rev Vis Sci. 2024. PMID: 39292551 Free PMC article. Review.
-
Role of AMIGO2 in cancer progression: Novel insights (Review).Oncol Lett. 2024 Jul 12;28(3):434. doi: 10.3892/ol.2024.14567. eCollection 2024 Sep. Oncol Lett. 2024. PMID: 39049987 Free PMC article. Review.
-
Molecular mechanism establishing the OFF pathway in vision.Nat Commun. 2025 Apr 18;16(1):3708. doi: 10.1038/s41467-025-59046-0. Nat Commun. 2025. PMID: 40251167 Free PMC article.
-
Astrocyte ensheathment of calyx-forming axons of the auditory brainstem precedes accelerated expression of myelin genes and myelination by oligodendrocytes.J Comp Neurol. 2024 Feb;532(2):e25552. doi: 10.1002/cne.25552. Epub 2023 Nov 2. J Comp Neurol. 2024. PMID: 37916792 Free PMC article.
-
Analysis of Free Circulating Messenger Ribonucleic Acids in Serum Samples from Late-Onset Spinal Muscular Atrophy Patients Using nCounter NanoString Technology.Cells. 2023 Sep 28;12(19):2374. doi: 10.3390/cells12192374. Cells. 2023. PMID: 37830588 Free PMC article.
References
-
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ (2004) Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 24:9598–9611. 10.1523/JNEUROSCI.1851-04.2004 - DOI - PMC - PubMed
-
- Chichilnisky EJ (2001) A simple white noise analysis of neuronal light responses. Network 12:199–213. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
