The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern
- PMID: 35169114
- PMCID: PMC8847371
- DOI: 10.1038/s41467-022-28354-0
The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern
Abstract
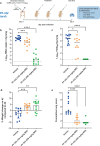
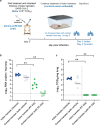
There is an urgent need for potent and selective antivirals against SARS-CoV-2. Pfizer developed PF-07321332 (PF-332), a potent inhibitor of the viral main protease (Mpro, 3CLpro) that can be dosed orally and that is in clinical development. We here report that PF-332 exerts equipotent in vitro activity against the four SARS-CoV-2 variants of concerns (VoC) and that it can completely arrest replication of the alpha variant in primary human airway epithelial cells grown at the air-liquid interface. Treatment of Syrian Golden hamsters with PF-332 (250 mg/kg, twice daily) completely protected the animals against intranasal infection with the beta (B.1.351) and delta (B.1.617.2) SARS-CoV-2 variants. Moreover, treatment of SARS-CoV-2 (B.1.617.2) infected animals with PF-332 completely prevented transmission to untreated co-housed sentinels.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- No 101003627 (SCORE project)/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- the Wellcome TrustGrant ref: 222489/Z/21/Z/Wellcome Trust (Wellcome)
- INV-00636/Bill and Melinda Gates Foundation (Bill & Melinda Gates Foundation)
- FWO (G0G4820N)/Fonds Wetenschappelijk Onderzoek (Research Foundation Flanders)
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

