UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism
- PMID: 35169125
- PMCID: PMC8847552
- DOI: 10.1038/s41419-022-04596-0
UBE2T-mediated Akt ubiquitination and Akt/β-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism
Abstract
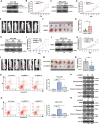
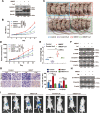
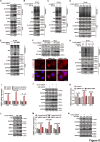
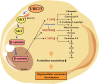
The oncogene protein ubiquitin-conjugating enzyme E2T (UBE2T) is reported to be upregulated in hepatocellular carcinoma (HCC) and correlated with poor clinical outcomes of HCC patients. However, the underlying mechanism by which UBE2T exerts its oncogenic function in HCC remains largely unexplored. In this study, in vitro and in vivo experiments suggested that UBE2T promoted HCC development including proliferation and metastasis. GSEA analysis indicated that UBE2T was positively correlated with pyrimidine metabolism, and LC/MS-MS metabolomics profiling revealed that the key products of pyrimidine metabolism were significantly increased in UBE2T-overexpressing cells. UBE2T overexpression led to the upregulation of several key enzymes catalyzing de novo pyrimidine synthesis, including CAD, DHODH, and UMPS. Moreover, the utilization of leflunomide, a clinically approved DHODH inhibitor, blocked the effect of UBE2T in promoting HCC progression. Mechanistically, UBE2T increased Akt K63-mediated ubiquitination and Akt/β-catenin signaling pathway activation. The disruption of UBE2T-mediated ubiquitination on Akt, including E2-enzyme-deficient mutation (C86A) of UBE2T and ubiquitination-site-deficient mutation (K8/14 R) of Akt impaired UBE2T's effect in upregulating CAD, DHODH, and UMPS. Importantly, we demonstrated that UBE2T was positively correlated with p-Akt, β-catenin, CAD, DHODH, and UMPS in HCC tumor tissues. In summary, our study indicates that UBE2T increases pyrimidine metabolism by promoting Akt K63-linked ubiquitination, thus contributing to HCC development. This work provides a novel insight into HCC development and a potential therapeutic strategy for HCC patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019;17:302–10. - PubMed
-
- Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology. 2018;67:381–400. - PubMed
-
- Liu LP, Yang M, Peng QZ, Li MY, Zhang YS, Guo YH, et al. UBE2T promotes hepatocellular carcinoma cell growth via ubiquitination of p53. Biochem Biophys Res Commun. 2017;493:20–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81903133/National Natural Science Foundation of China (National Science Foundation of China)
- 81902478/National Natural Science Foundation of China (National Science Foundation of China)
- 82073343/National Natural Science Foundation of China (National Science Foundation of China)
- 81972897/National Natural Science Foundation of China (National Science Foundation of China)
- 82073394/National Natural Science Foundation of China (National Science Foundation of China)
- 2020M672736/China Postdoctoral Science Foundation
- 2021A1515012151/Medical Science and Technology Foundation of Guangdong Province (Science and Technology Foundation of Guangdong Province)
- 2017A030310105/Medical Science and Technology Foundation of Guangdong Province (Science and Technology Foundation of Guangdong Province)
- 2019A1515011436/Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation)
- No.201906010087/Guangzhou Municipal Science and Technology Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

